Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help in solving part 4 and questions 1,2,&3 for chemistry lab!! the datas is in the picture from lab 2. Complete the
can you please help in solving part 4 and questions 1,2,&3 for chemistry lab!! 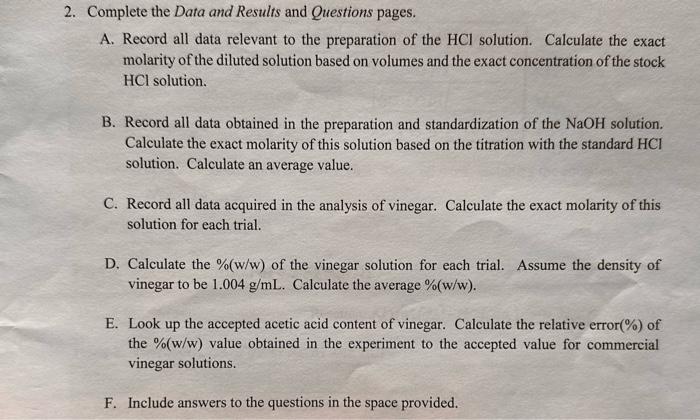


the datas is in the picture from lab
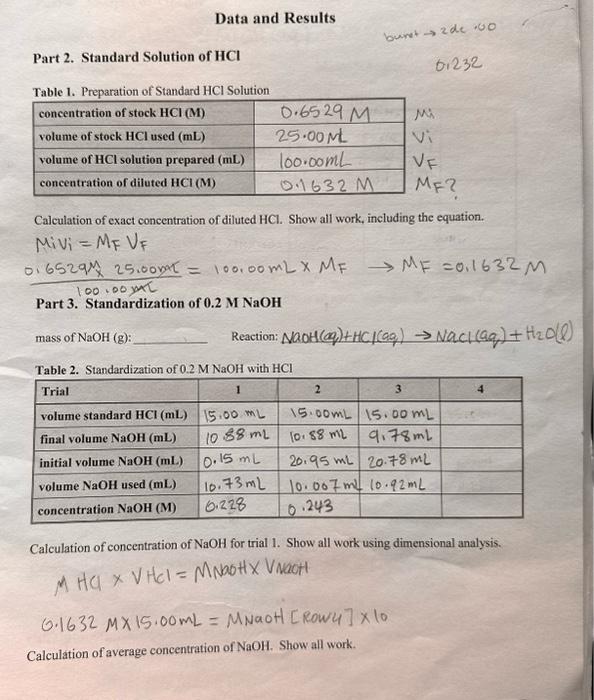
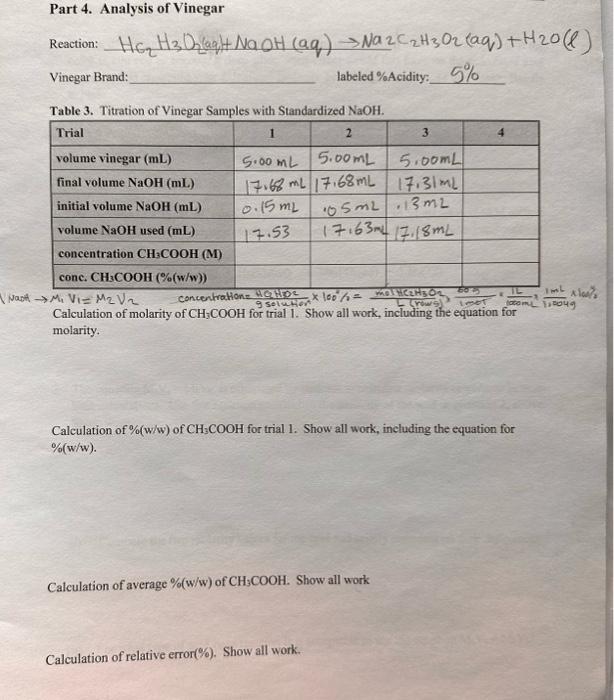
2. Complete the Data and Results and Questions pages. A. Record all data relevant to the preparation of the HCl solution. Calculate the exact molarity of the diluted solution based on volumes and the exact concentration of the stock HCl solution. B. Record all data obtained in the preparation and standardization of the NaOH solution. Calculate the exact molarity of this solution based on the titration with the standard HCl solution. Calculate an average value. C. Record all data acquired in the analysis of vinegar. Calculate the exact molarity of this solution for each trial. D. Calculate the %(w/w) of the vinegar solution for each trial. Assume the density of vinegar to be 1.004g/mL. Calculate the average %(w/w). E. Look up the accepted acetic acid content of vinegar. Calculate the relative error(\%) of the %(w/w) value obtained in the experiment to the accepted value for commercial vinegar solutions. F. Include answers to the questions in the space provided. Data and Results Part 2. Standard Solution of HCl Table 1. Preparation of Standard HCl Solution Calculation of exact concentration of diluted HCl. Show all work, including the equation. MiVi=MFVF 100.00mL0.6529M25.00NaL=100.00mLMFMF=0.1632M Part 3. Standardization of 0.2MNaOH mass of NaOH(g) : Reaction: NaOH(q)+HCl(aq)NaCl(aqq)+H2O(l) Tahla 2 Standardization of 02MNaOH with HCl Calculation of concentration of NaOH for trial 1. Show all work using dimensional analysis. M Ha VHCI=MNa+H Vaot 0.1632M15.00mL= MNaotl [ RowU ]10 Calculation of average concentration of NaOH. Show all work. Part 4. Analysis of Vinegar Vinegar Brand: labeled \%Acidity: 5% Tahle 3. Titration of Vineoar Samnles with Standardized NaOH Calculation of molarity of CH3COOH for trial 1 . Show all work, including the equation for molarity. Calculation of %(w/w) of CH3COOH for trial 1. Show all work, including the equation for %(w/w) Calculation of average %(w/w) of CH3COOH. Show all work Calculation of relative error (%). Show all work. 1. During the titration, the goal was to achieve a faint pink solution color at the endpoint. If the color is allowed to become a deep pink, what effect would this have on the calculated acid molarity; that is, would your calculated molarity be too high, too low, or the same? Briefly explain. 2. The titration of a 25.00mL sample of phosphoric acid required 34.52mL of 0.3840M sodium hydroxide to reach the endpoint. Calculate the molarity of the acid using dimensional analysis. Show all work. 3NaOH(aq)+H3PO4(aq)Na3PO4(aq)+3H2O(c) 3. The equation M2Va=MbVb is often used by students to calculate molarity or volume in acidbase calculations. Use this equation to calculate the molarity of the acid in question 2 , above. Show work. (i) Compare the two molarity values for the acid and briefly comment on their differences. (ii) For what type of acid-base reaction is it acceptable to use the equation MaVa=MbVs Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


