Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help me answer i cannot figure these out The equilibrium constant, K, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) An
can you please help me answer i cannot figure these out 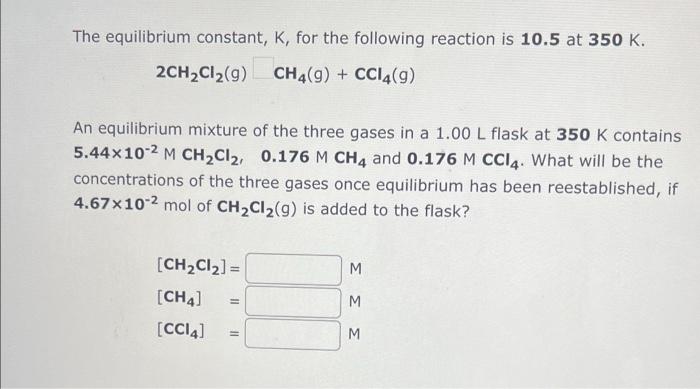
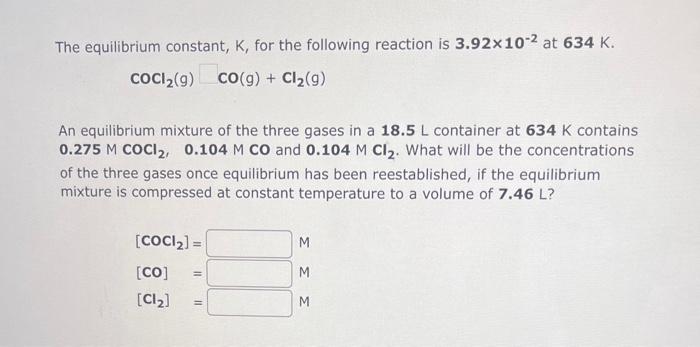
The equilibrium constant, K, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) An equilibrium mixture of the three gases in a 1.00L flask at 350K contains 5.44102MCH2Cl2,0.176MCH4 and 0.176MCCl4. What will be the concentrations of the three gases once equilibrium has been reestablished, if 4.67 102mol of CH2Cl2(g) is added to the flask? The equilibrium constant, K, for the following reaction is 3.92102 at 634K. COCl2(g)CO(g)+Cl2(g) An equilibrium mixture of the three gases in a 18.5L container at 634K contains 0.275MCOCl2,0.104MCO and 0.104MCl2. What will be the concentrations of the three gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 7.46L 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


