Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help me solve tha question below ? i couldn't get it .. thank you See bubbling instructions on the back of this
can you please help me solve tha question below ? i couldn't get it .. thank you 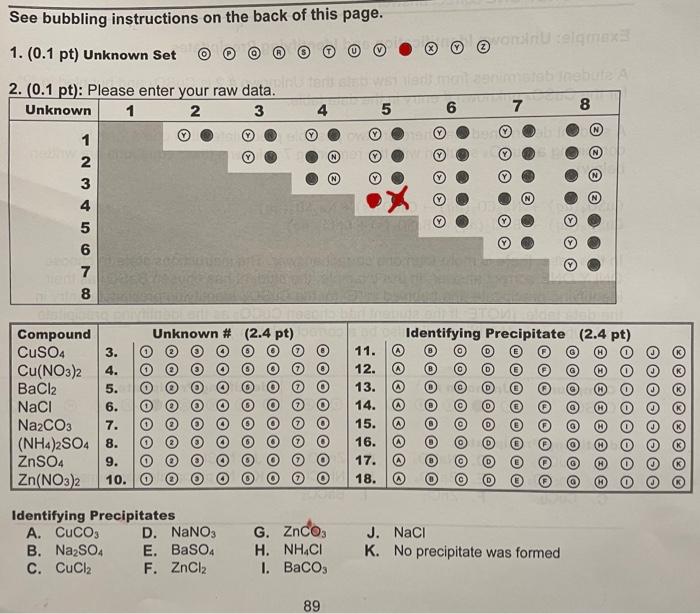


See bubbling instructions on the back of this page. 1. (0.1 pt) Unknown Set () ( ) () () (T) (2) Identifying Precipitates A. CuCO3 D. NaNO3 G. ZnCO3 J. NaCl B. Na2SO4 E. BaSO4 H. NH4Cl K. No precipitate was formed C. CuCl2 F. ZnCl2 I. BaCO3 Bubbling information for data tables on Exp. 9 Grade Sheet Raw Data Set: Indicate which unknown combinations created a precipitate by filling in the appropriate bubble in the table provided. Fill in if a precipitate formed or if a precipitate did not form. Unknown Identification and Identifying Precipitates Identify unknown by bubbling in the correct unknown \#. Identify which precipitate was formed by bubbling in the correct letter from the "Identifying Precipitates" list. There may be more than one correct answer. Please choose ONLY ONE correct answer. Example: Unknown Identification and Identifying Precipitates A student determines from their raw data that Unknown \# 2 is CuSO4 and bubbled in "2" for the CuSO4 unknown \# entry in the data table. A student determined that there are two possible precipitates that could form by combining a CuSO4 solution with the remaining 7 solutions (their equations are written below). CuSO4(aq)+Na2CO3(aq)CuCO3(s)+Na2SO4(aq)CuSO4(aq)+BaCl2(aq)BaSO4(s)+CuCl2(aq) In this case, there is more than one correct answer. The instructions state to choose ONLY ONE correct answer. In this example, the student has chosen BaSO4 as their identifying precipitate and bubbled in "E" for the CuSO4 identifying precipitate entry in the data table. (NOTE: If the student had chosen CuCO3 as the identifying precipitate and bubbled in " A ", this would also be a correct solution.) Experiment \# 9 - Using Solubilities to Identify 8 Unknown Solutions The solubilities of ionic compounds in water vary over a wide range. Some ionic compounds such as sodium chloride are very soluble in water while others such as calcium sulfate are not very soluble in water. This is exploited by the chemical and mining industries for the isolation and purification of specific elements from complex mixtures. The reaction between water soluble salts in which the water soluble reagents give an insoluble product represents a precipitation reaction. For example, the reaction between aqueous solutions of NaClandAgNO3, shown in Equation 9.1, is a precipitation reaction due to the relative insolubility of AgCl. NaCl(aq)+AgNO3(aq)AgCl(s)+NaNO3(aq) In contrast, combining aqueous solutions of NaCl and KNO3, shown in Equation 9.2, would not yield a precipitate.X NaCl(aq)+KNO3(aq)KCl(aq)+NaNO3(aq) In general, the solubilities of ionic compounds follow several broad rules which can allow one to predict relative solubilities. As outlined in Table 9.1, salts containing the nitrate (NO3)anion are commonly soluble in water. Similarly, most salts containing the heavier halides (Cl,Br,r)and sulfates (SOt2) are water soluble with the exception of some salts featuring Ag+,Hgz2,Pb2,Sr2+, and Ba2+. Conversely, a majority of salts containing S2,CO32, or PO43 anions are insoluble in water, although some exceptions do exist. All common salts of the alkali metal cations and of ammonium are soluble in water. In this experiment, you will use differences in solubilities to distinguish between 8 different unknown solutions. You will perform a series of spot tests, in which one drop from each of two solutions is mixed and the occurrence or lack of a precipitate recorded. An additional tool is the fact that some metal ions give solutions a characteristic color (e.g., most Cu2+ solutions are blue. Ni2+ solutions are green); whereas others are colorless (e.g., most Ba2+,Zn2+,Na+, and NH+solutions). See bubbling instructions on the back of this page. 1. (0.1 pt) Unknown Set () ( ) () () (T) (2) Identifying Precipitates A. CuCO3 D. NaNO3 G. ZnCO3 J. NaCl B. Na2SO4 E. BaSO4 H. NH4Cl K. No precipitate was formed C. CuCl2 F. ZnCl2 I. BaCO3 Bubbling information for data tables on Exp. 9 Grade Sheet Raw Data Set: Indicate which unknown combinations created a precipitate by filling in the appropriate bubble in the table provided. Fill in if a precipitate formed or if a precipitate did not form. Unknown Identification and Identifying Precipitates Identify unknown by bubbling in the correct unknown \#. Identify which precipitate was formed by bubbling in the correct letter from the "Identifying Precipitates" list. There may be more than one correct answer. Please choose ONLY ONE correct answer. Example: Unknown Identification and Identifying Precipitates A student determines from their raw data that Unknown \# 2 is CuSO4 and bubbled in "2" for the CuSO4 unknown \# entry in the data table. A student determined that there are two possible precipitates that could form by combining a CuSO4 solution with the remaining 7 solutions (their equations are written below). CuSO4(aq)+Na2CO3(aq)CuCO3(s)+Na2SO4(aq)CuSO4(aq)+BaCl2(aq)BaSO4(s)+CuCl2(aq) In this case, there is more than one correct answer. The instructions state to choose ONLY ONE correct answer. In this example, the student has chosen BaSO4 as their identifying precipitate and bubbled in "E" for the CuSO4 identifying precipitate entry in the data table. (NOTE: If the student had chosen CuCO3 as the identifying precipitate and bubbled in " A ", this would also be a correct solution.) Experiment \# 9 - Using Solubilities to Identify 8 Unknown Solutions The solubilities of ionic compounds in water vary over a wide range. Some ionic compounds such as sodium chloride are very soluble in water while others such as calcium sulfate are not very soluble in water. This is exploited by the chemical and mining industries for the isolation and purification of specific elements from complex mixtures. The reaction between water soluble salts in which the water soluble reagents give an insoluble product represents a precipitation reaction. For example, the reaction between aqueous solutions of NaClandAgNO3, shown in Equation 9.1, is a precipitation reaction due to the relative insolubility of AgCl. NaCl(aq)+AgNO3(aq)AgCl(s)+NaNO3(aq) In contrast, combining aqueous solutions of NaCl and KNO3, shown in Equation 9.2, would not yield a precipitate.X NaCl(aq)+KNO3(aq)KCl(aq)+NaNO3(aq) In general, the solubilities of ionic compounds follow several broad rules which can allow one to predict relative solubilities. As outlined in Table 9.1, salts containing the nitrate (NO3)anion are commonly soluble in water. Similarly, most salts containing the heavier halides (Cl,Br,r)and sulfates (SOt2) are water soluble with the exception of some salts featuring Ag+,Hgz2,Pb2,Sr2+, and Ba2+. Conversely, a majority of salts containing S2,CO32, or PO43 anions are insoluble in water, although some exceptions do exist. All common salts of the alkali metal cations and of ammonium are soluble in water. In this experiment, you will use differences in solubilities to distinguish between 8 different unknown solutions. You will perform a series of spot tests, in which one drop from each of two solutions is mixed and the occurrence or lack of a precipitate recorded. An additional tool is the fact that some metal ions give solutions a characteristic color (e.g., most Cu2+ solutions are blue. Ni2+ solutions are green); whereas others are colorless (e.g., most Ba2+,Zn2+,Na+, and NH+solutions) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


