Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help me with #4 1. Using the video above, record the mass and volume a b The second trial starts with some
can you please help me with #4 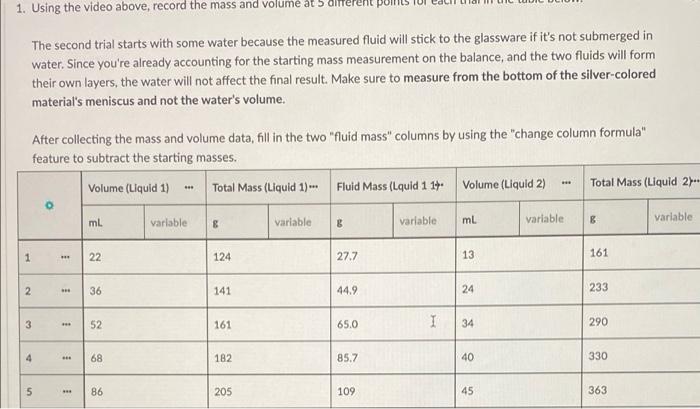
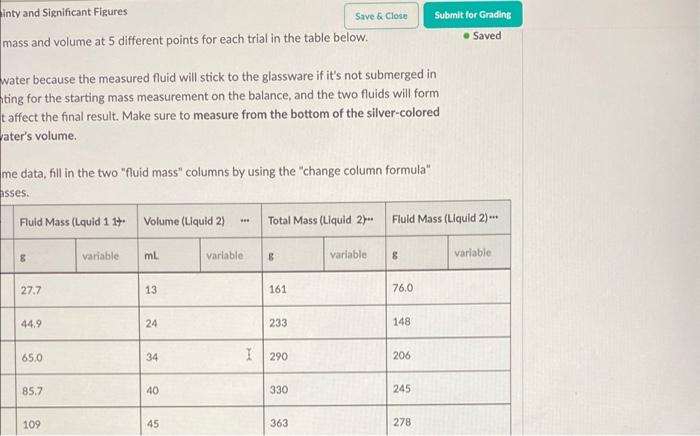
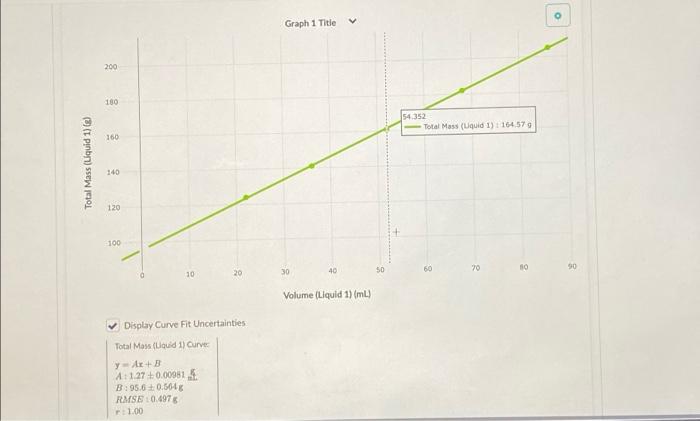
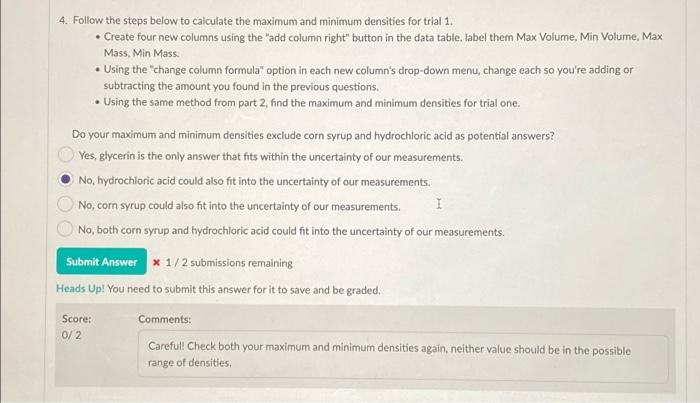
1. Using the video above, record the mass and volume a b The second trial starts with some water because the measured fluid will stick to the glassware if it's not submerged in water. Since you're already accounting for the starting mass measurement on the balance, and the two fluids will form their own layers, the water will not affect the final result. Make sure to measure from the bottom of the silver-colored material's meniscus and not the water's volume. After collecting the mass and volume data, fill in the two "fluid mass" columns by using the "change column formula" feature to subtract the starting masses. Volume (Liquid 1) - Total Mass (Liquid 1) - Fluid Mass (Lquid 1 14. Volume (Liquid 2) Total Mass (Liquid 2. 0 ml variable variable 8 variable ml B Variable 8 variable 1 22 124 *** 27.7 13 161 N 36 141 44.9 24 233 3 3 52 161 65.0 I 34 290 4 68 182 85.7 40 330 5 86 1 205 109 45 363 inty and Significant Figures Save & Close mass and volume at 5 different points for each trial in the table below. Submit for Grading Saved water because the measured fluid will stick to the glassware if it's not submerged in ting for the starting mass measurement on the balance, and the two fluids will form t affect the final result. Make sure to measure from the bottom of the silver-colored ater's volume. me data, fill in the two "fluid mass" columns by using the change column formula" asses. Fluid Mass (Lquid 1 17 Volume (Liquid 2) Total Mass (Liquid 2- Fluid Mass (Liquid 2) 8 variable ml variable 8 variable 8 variable 27.7 13 161 76,0 44.9 24 233 148 65.0 34 1 290 206 85.7 40 330 245 109 45 363 278 o Graph 1 Title 200 180 54.352 Total Mass (Uquid 1) 1164.579 160 Total Mass (Liquid 1)(e) 140 120 100 40 50 60 20 30 90 30 20 10 Volume (Liquid 1) (L) Display Curve Fit Uncertainties Total Moss (Loud 1) Curve y Art 41.27 +0.00951. B:95.6 0.504 FMSE10.4976 1.00 4. Follow the steps below to calculate the maximum and minimum densities for trial 1. Create four new columns using the add column right" button in the data table label them Max Volume, Min Volume, Max Mass, Min Mass. . Using the "change column formula" option in each new column's drop-down menu, change each so you're adding or subtracting the amount you found in the previous questions. . Using the same method from part 2, find the maximum and minimum densities for trial one: Do your maximum and minimum densities exclude corn syrup and hydrochloric acid as potential answers? Yes, glycerin is the only answer that fits within the uncertainty of our measurements. No, hydrochloric acid could also fit into the uncertainty of our measurements. No, corn syrup could also fit into the uncertainty of our measurements. 1 No, both corn syrup and hydrochloric acid could fit into the uncertainty of our measurements. Submit Answer * 1/2 submissions remaining Heads Up! You need to submit this answer for it to save and be graded. Score: Comments: Careful! Check both your maximum and minimum densities again, neither value should be in the possible range of densities 0/2 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


