Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you please solve all three questions? thank you. i need your help. Use the References to access important values if needed for this question.
Can you please solve all three questions? thank you. i need your help. 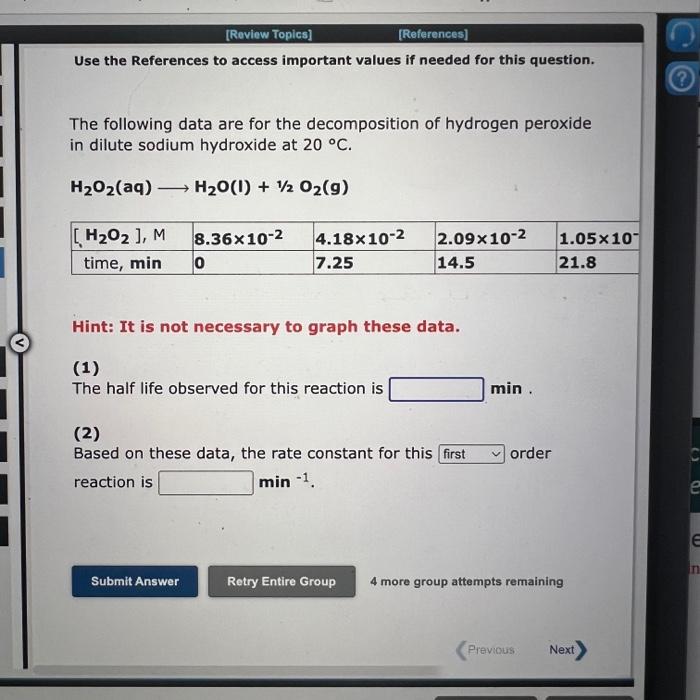
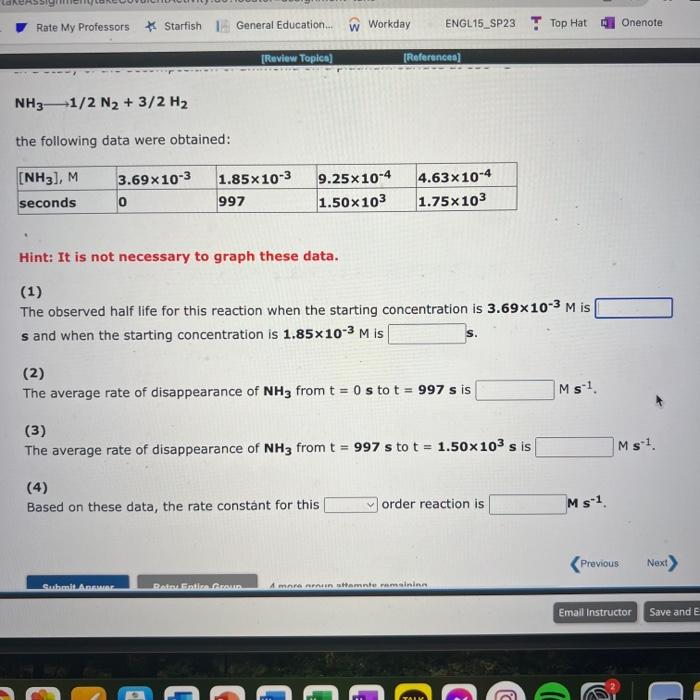
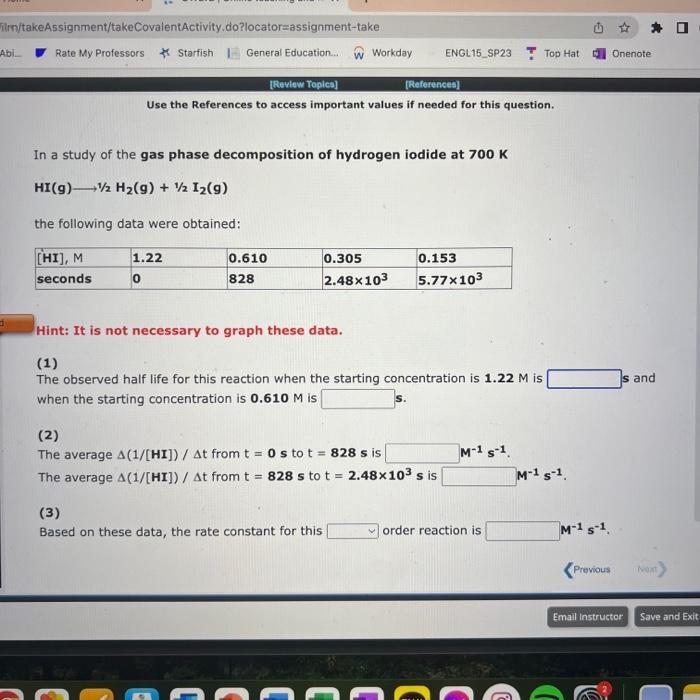
Use the References to access important values if needed for this question. The following data are for the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20C. H2O2(aq)H2O(I)+1/2O2(g) Hint: It is not necessary to graph these data. (1) The half life observed for this reaction is (2) Based on these data, the rate constant for this order reaction is min1. NH31/2N2+3/2H2 the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 3.69103M is s and when the starting concentration is 1.85103M is s. (2) The average rate of disappearance of NH3 from t=0s to t=997s is Ms1 (3) The average rate of disappearance of NH3 from t=997s to t=1.50103s is Ms1 (4) Based on these data, the rate constant for this order reaction is Ms1. Use the References to access important values if needed for this question. In a study of the gas phase decomposition of hydrogen iodide at 700K HI(g)1/2H2(g)+1/2I2(g) the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 1.2M is 5 and when the starting concentration is 0.610M is 5. (2) The average (1/[ HI ])/t from t=0s to t=828s is M1s1 The average (1/[HI])/t from t=828s to t=2.48103s is M1s1. (3) Based on these data, the rate constant for this order reaction is M1s1 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


