Can you please solve the question b, c, d and e for me please?
Given that:
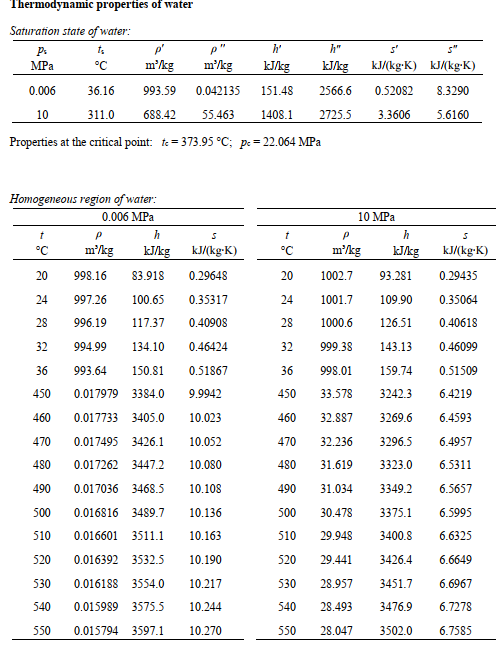
Task 3 (21) In a heat recovery boiler (steam generator), the exhaust gases of a gas turbine process are used to evaporate water in an isobaric steam power process at the pressure pa = 10 MPa. During this process, the temperature of the water increases from t2 = 28 C to 3=510 C. The superheated steam is then expanded into the wet steam region and to the condenser pressure p = 0.006 MPa via an irreversible adiabatic turbine (1.1 = 90%). The isobaric condenser supercools the liquid by AT = 10 K before it is fed to the irreversibly adiabatic boiler feed pump. The exhaust gases of the gas turbine process enter the isobaric boiler with the mass flow ritesh = 380 kg/s at a temperature of tes = 520 C, and leave it with the temperature tout = 190 C. The exhaust gases can be considered ideal gases with a constant heat capacity of Cpath = 1.31 kJ/(kg.K). Exhaust gas with a Exhaust gas with boiler 3 P 1 condenser a) Illustrate a qualitatively correct T-s-diagram of the steam power process. (3) b) Determine the mass flow m, circulating in the steam power process. c) Determine the effective (useful) power of the steam power process Pae (8) d) Determine the thermal efficiency np.comb for the combined process of gas and steam tur- bine, in the case of Px.cz=-113.7 MW and Trib.G1 = 34.4% for the gas turbine process. (2) e) Determine the exergy loss AE, in the hear recovery boiler for an ambient temperature of to = 20 C. Thermodynamic properties of water Saturation state of water: P. ts p' MPa C m'/kg m'/kg 0.006 36.16 993.59 0.042135 h kJ/kg h" kJ/kg s' kJ/(kg:K) kJ/kg-K) 151.48 2566.6 0.52082 8.3290 10 311.0 688.42 55.463 1408.1 2725.5 3.3606 5.6160 Properties at the critical point: te = 373.95 C; p= 22.064 MPa Homogeneous region of water: 0.006 MPa h m'/kg kJ/kg 1 P S 10 MPa P h m'/kg IJ/kg s 7 C kJ/kg-K) kJ/(kg:K) 20 998.16 83.918 0.29648 20 1002.7 93.281 0.29435 24 997.26 100.65 24 1001.7 109.90 0.35064 28 996.19 117.37 28 1000.6 126.51 0.40618 32 994.99 134.10 0.35317 0.40908 0.46424 0.51867 9.9942 32 999.38 143.13 0.46099 36 993.64 150.81 36 998.01 159.74 0.51509 450 0.017979 3384.0 450 33.578 3242.3 6.4219 460 0.017733 3405.0 10.023 460 32.887 3269.6 6.4593 470 470 32.236 3296.5 6.4957 480 480 31.619 3323.0 6.5311 490 490 31.034 3349.2 6.5657 0.0174953426.1 0.017262 34472 0.017036 3468.5 0.016816 3489.7 0.016601 3511.1 0.016392 3532.5 0.016188 3554.0 10.052 10.080 10.108 10.136 10.163 10.190 500 30.478 3375.1 6.5995 500 510 510 29.948 3400.8 6.6325 520 520 29.441 3426.4 6.6649 530 10.217 530 28.957 3451.7 6.6967 540 0.0159893575.5 10.244 540 28.493 3476.9 6.7278 550 0.0157943597.1 10.270 550 28.047 3502.0 6.7585








