Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you plz try to answer all the questions Thanks Balance the following unbalanced reaction using the lowest whole number coefficients. H(aq) + CIO2 (aq)
Can you plz try to answer all the questions 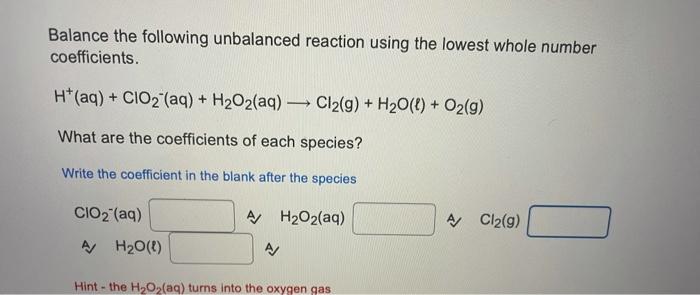
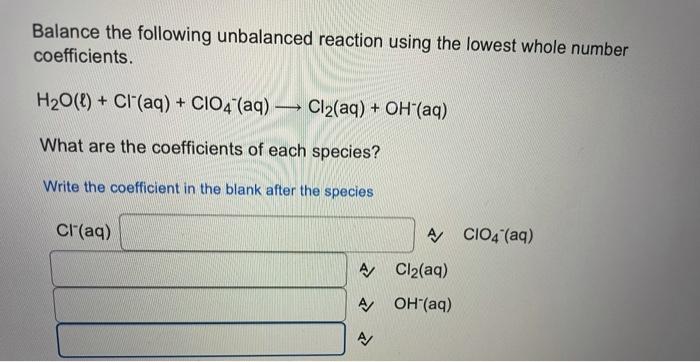
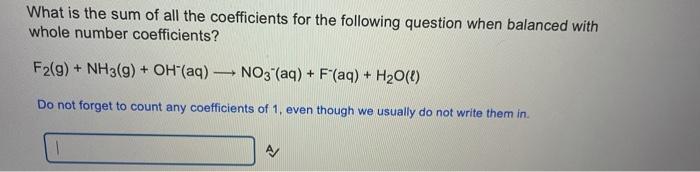
Balance the following unbalanced reaction using the lowest whole number coefficients. H(aq) + CIO2 (aq) + H2O2(aq) Cl2(g) + H2O(l) + O2(g) What are the coefficients of each species? Write the coefficient in the blank after the species CIO2 (aq) A H2O2(aq) AJ Cl2(9) A/ H20(1) A Hint - the H2O2(aq) turns into the oxygen gas Balance the following unbalanced reaction using the lowest whole number coefficients. H2O(l) + Cl(aq) + CIO4 (aq) Cl2(aq) + OH"(aq) What are the coefficients of each species? Write the coefficient in the blank after the species Cl(aq) A CIO4 (aq) A Cl2(aq) A OH(aq) What is the sum of all the coefficients for the following question when balanced with whole number coefficients? F2(g) + NH3(g) + OH(aq) NO3- (aq) + F"(aq) + H20() Do not forget to count any coefficients of 1, even though we usually do not write them in A/ Thanks



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


