Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you solve the 'b part' of this question? 1. A small reaction bomb with a sensitive pressure measuring device is flushed out and filled
can you solve the 'b part' of this question? 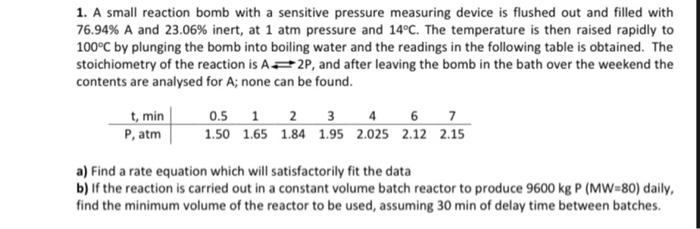
1. A small reaction bomb with a sensitive pressure measuring device is flushed out and filled with 76.94% A and 23.06% inert, at 1 atm pressure and 14C. The temperature is then raised rapidly to 100C by plunging the bomb into boiling water and the readings in the following table is obtained. The stoichiometry of the reaction is A2P, and after leaving the bomb in the bath over the weekend the contents are analysed for A; none can be found. t, min 0.5 1 2 3 4 6 7 P, atm 1.50 1.65 1.84 1.95 2.025 2.12 2.15 a) Find a rate equation which will satisfactorily fit the data b) If the reaction is carried out in a constant volume batch reactor to produce 9600 kg P (MW=80) daily, find the minimum volume of the reactor to be used, assuming 30 min of delay time between batches 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


