Question
A vital component used as a Grignard reagent in organic synthesis is found in the lab. Determine its empirical and molecular formula if its
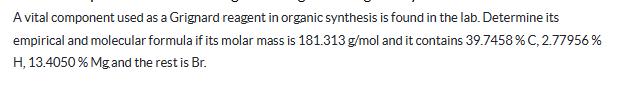
A vital component used as a Grignard reagent in organic synthesis is found in the lab. Determine its empirical and molecular formula if its molar mass is 181.313 g/mol and it contains 39.7458 % C, 2.77956% H, 13.4050 % Mg and the rest is Br.
Step by Step Solution
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Climate change a pressing global issue has garnered significant attention in recent years d...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Financial Reporting and Analysis
Authors: David Alexander, Anne Britton, Ann Jorissen
5th edition
978-1408032282, 1408032287, 978-1408075012
Students also viewed these Business Communication questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



