Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you write the solution with python? (25pts) van der Waals Equation The equation of state PV=nRT for ideal gases approximates the state of real
Can you write the solution with python?
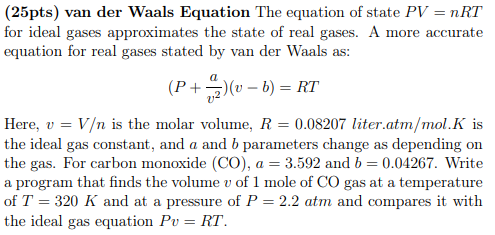 (25pts) van der Waals Equation The equation of state PV=nRT for ideal gases approximates the state of real gases. A more accurate equation for real gases stated by van der Waals as: (P+v2a)(vb)=RT Here, v=V is the molar volume, R=0.08207 liter.atm /mol.K is the ideal gas constant, and a and b parameters change as depending on the gas. For carbon monoxide (CO), a=3.592 and b=0.04267. Write a program that finds the volume v of 1 mole of CO gas at a temperature of T=320K and at a pressure of P=2.2atm and compares it with the ideal gas equation Pv=RT
(25pts) van der Waals Equation The equation of state PV=nRT for ideal gases approximates the state of real gases. A more accurate equation for real gases stated by van der Waals as: (P+v2a)(vb)=RT Here, v=V is the molar volume, R=0.08207 liter.atm /mol.K is the ideal gas constant, and a and b parameters change as depending on the gas. For carbon monoxide (CO), a=3.592 and b=0.04267. Write a program that finds the volume v of 1 mole of CO gas at a temperature of T=320K and at a pressure of P=2.2atm and compares it with the ideal gas equation Pv=RT Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


