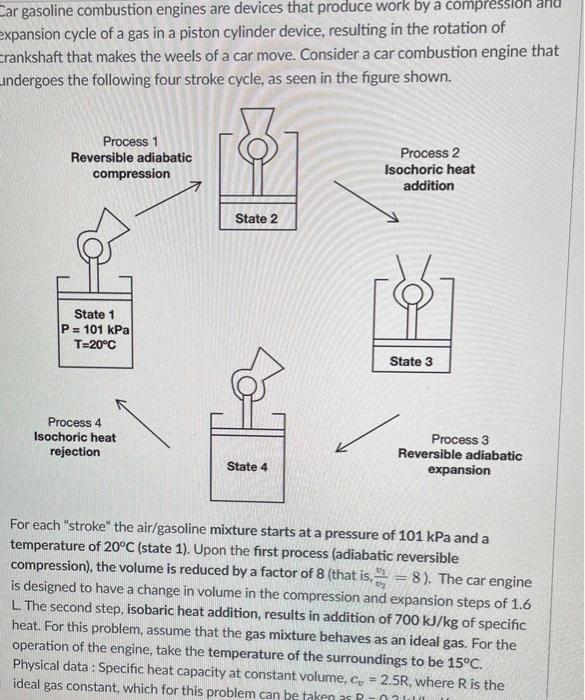
Car gasoline combustion engines are devices that produce work by a compression and expansion cycle of a gas in a piston cylinder device, resulting in the rotation of crankshaft that makes the weels of a car move. Consider a car combustion engine that undergoes the following four stroke cycle, as seen in the figure shown. For each "stroke" the air/gasoline mixture starts at a pressure of 101kPa and a temperature of 20C (state 1). Upon the first process (adiabatic reversible compression), the volume is reduced by a factor of 8 (that is, v2v1=8 ). The car engine is designed to have a change in volume in the compression and expansion steps of 1.6 L. The second step, isobaric heat addition, results in addition of 700kJ/kg of specific heat. For this problem, assume that the gas mixture behaves as an ideal gas. For the operation of the engine, take the temperature of the surroundings to be 15C. Physical data: Specific heat capacity at constant volume, cv=2.5R, where R is the ideal gas constant, which for this problem can Determine the net specific work produced by the cycle. Express your answer in kW/kg. Question 2 Determine the net work produced by the cycle. Express your answer in kW. Question 3 1pts Determine the thermal efficiency of this cycle. You may express your answer as a percentage of a decimal. Question 4 1 pts Calculate the net generation of specific entropy of the system for the cycle (Ssys). Take the system as the gas mixture. Express your answer in kJ/kgK. Calculate the net generation of specific entropy of the university (Suniv) for the cycle. Express your answer in kJ/kgK. Hint: You may obtain a value you do not expect. Question 6 1pts The cycle presented in this problem contains steps that are reversible. In reality, car engines are not reversible. Based on this statement, what can we conclude? Select all that apply. The work generated by the reversible engine studied represents the maximum amount of work that is possible. The work generated by the reversible engine studied represents the minimum amount of work that is possible. The work generated by a real (non-reversible) engine would be larger compared to the reversible engine. The work generated by a real (non-reversible) engine would be smaller compared to the reversible engine