Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Carbon has the electron configuration 1s 2s2p. The two unpaired electrons in the n = 2 level suggests that carbon will form two bonds.
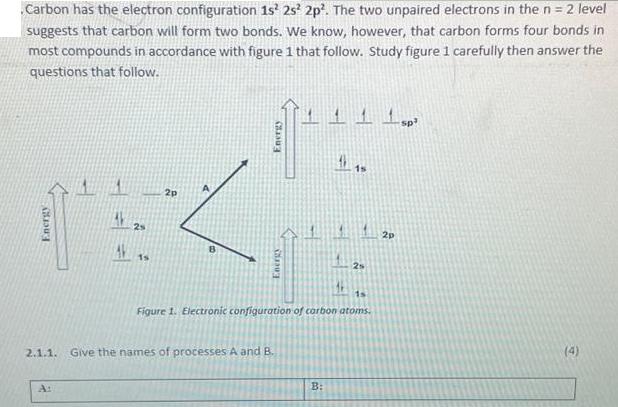
Carbon has the electron configuration 1s 2s2p. The two unpaired electrons in the n = 2 level suggests that carbon will form two bonds. We know, however, that carbon forms four bonds in most compounds in accordance with figure 1 that follow. Study figure 1 carefully then answer the questions that follow. Energy 2s 15 2p Energy Energy 2.1.1. Give the names of processes A and B. 115 1s Figure 1. Electronic configuration of carbon atoms. B: 2 1 sp 2p (4)
Step by Step Solution
★★★★★
3.34 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
4 Hybridization and Promotion of electrons respectively 212 What are the numbers of electrons involv...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


