Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Carefully study the following intramolecular Diels-Alder reaction scheme and answer the questions that follow. PLEASE NOTE: The CHO group and the benzene ring are
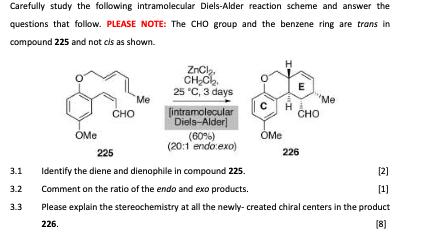
Carefully study the following intramolecular Diels-Alder reaction scheme and answer the questions that follow. PLEASE NOTE: The CHO group and the benzene ring are trans in compound 225 and not cls as shown. 3.1 3.2 3.3 OMe CHO Me ZnCl CHC 25 C, 3 days [intramolecular Diels-Alder] (60%) (20:1 endo:exo) H OMe E CHO Me 225 Identify the diene and dienophile in compound 225. Comment on the ratio of the endo and exo products. Please explain the stereochemistry at all the newly-created chiral centers in the product 226. [8] 226 [2] [1] Carefully study the following intramolecular Diels-Alder reaction scheme and answer the questions that follow. PLEASE NOTE: The CHO group and the benzene ring are trans in compound 225 and not cls as shown. 3.1 3.2 3.3 OMe CHO Me ZnCl CHC 25 C, 3 days [intramolecular Diels-Alder] (60%) (20:1 endo:exo) H OMe E CHO Me 225 Identify the diene and dienophile in compound 225. Comment on the ratio of the endo and exo products. Please explain the stereochemistry at all the newly-created chiral centers in the product 226. [8] 226 [2] [1]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer Diene part Me Dienophile part ome 2 the dien...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


