Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Density of Common Gases at 0C Gas Density of Gas (g/L) Carbon Dioxide 1.799 Nitrogen 1.251 Oxygen 1.429 0.179 1.784 Helium Argon Assuming no
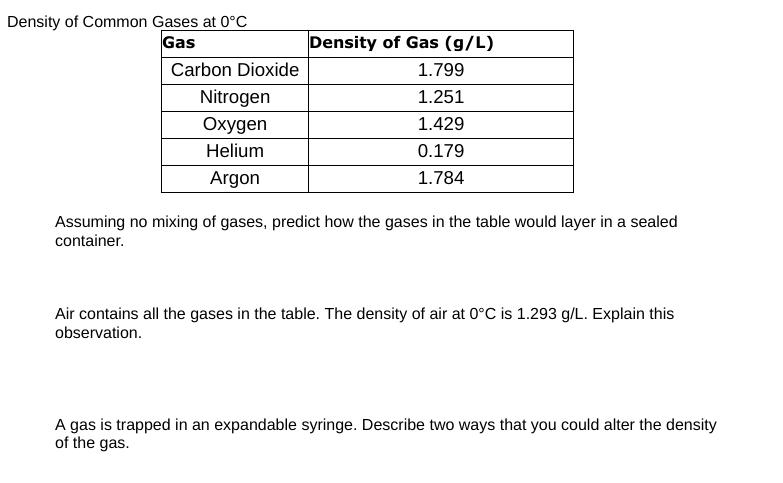

Density of Common Gases at 0C Gas Density of Gas (g/L) Carbon Dioxide 1.799 Nitrogen 1.251 Oxygen 1.429 0.179 1.784 Helium Argon Assuming no mixing of gases, predict how the gases in the table would layer in a sealed container. Air contains all the gases in the table. The density of air at 0C is 1.293 g/L. Explain this observation. A gas is trapped in an expandable syringe. Describe two ways that you could alter the density of the gas. The density of solid carbon dioxide is 1.562 g/cm. Convert this to grams per litre and compare this quantity to that of gaseous carbon dioxide. Use the particle model to illustrate the difference in density between the solid and gaseous state of carbon dioxide. S The concentration of gases is sometimes expressed using units of parts per million (ppm) and parts per billion (ppb). A concentration of carbon monoxide gas of 3200 ppm (3196 mg/L) can cause unconsciousness and death in less than 30 min. a) What is this concentration of gas in mol/L? b) How many molecules of carbon monoxide gas would exist in a cubic metre of this air?
Step by Step Solution
★★★★★
3.26 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


