Answered step by step
Verified Expert Solution
Question
1 Approved Answer
carry out an energy balance on the isothermal reactor being used to produce methyl acetate . how much heat is added/removed from reactor to maintain
carry out an energy balance on the isothermal reactor being used to produce methyl acetate . how much heat is added/removed from reactor to maintain isothermal function 

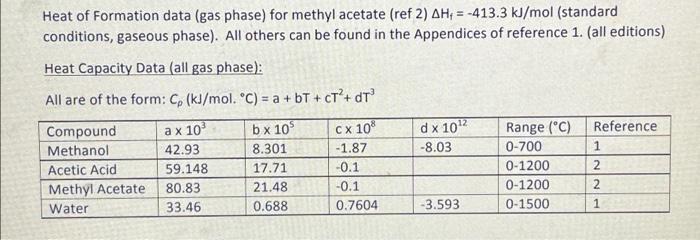
CH3OH + CH3COOH CH,COOCH3 +H2O The reaction occurs in the gaseous phase with a reactor temperature of between 300 and 400C. Prior to reaction, the mixture of gaseous methanol and acetic acid is heated from 150C to the reaction temperature in a heat exchanger, before entering the reactor. The reactor is maintained at the required temperature to ensure that methanol conversion is maintained. For an input of 150 mol/h of methanol, 100 mol/h acetic acid with a methanol conversion of 40%, the energy input required from the heat exchanger to raise the input stream temperature from 150C to 350C, the reactor output molar flowrate and mole fractions, and the energy added or removed from the reactor for isothermal operation at 350C. Heat of Formation data (gas phase) for methyl acetate (ref 2) AH = -413.3 kJ/mol (standard conditions, gaseous phase). All others can be found in the Appendices of reference 1. (all editions) Heat Capacity Data (all gas phase): All are of the form: C (kJ/mol. C) = a +bT + CT + DT a x 10 CX 10% d x 1012 -8.03 Reference 1 Compound Methanol Acetic Acid Methyl Acetate Water b x 10 8.301 17.71 21.48 0.688 42.93 59.148 80.83 33.46 2 Range (C) 0-700 0-1200 0-1200 0-1500 -1.87 -0.1 -0.1 0.7604 2 -3.593 1 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


