Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Catalyst effectiveness factor ()=0.652670905 Reaction rate of ammonia (RNH3)=56791.1128kmol/m3hr Area of reactor (A)=706m2 Molar flowrate of Nitrogen (FN2)=135.43kmol/hr X(0)=0 L(0)=0 Question: Solve dLdX=2FN2RNH3A using 4th
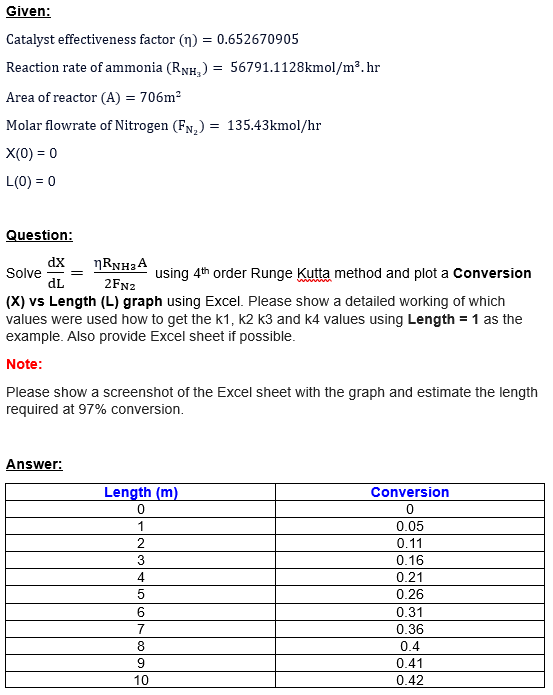 Catalyst effectiveness factor ()=0.652670905 Reaction rate of ammonia (RNH3)=56791.1128kmol/m3hr Area of reactor (A)=706m2 Molar flowrate of Nitrogen (FN2)=135.43kmol/hr X(0)=0 L(0)=0 Question: Solve dLdX=2FN2RNH3A using 4th order Runge Kutta method and plot a Conversion (X) vs Length (L) graph using Excel. Please show a detailed working of which values were used how to get the k1,k2k3 and k4 values using Length =1 as the example. Also provide Excel sheet if possible. Note: Please show a screenshot of the Excel sheet with the graph and estimate the length required at 97% conversion
Catalyst effectiveness factor ()=0.652670905 Reaction rate of ammonia (RNH3)=56791.1128kmol/m3hr Area of reactor (A)=706m2 Molar flowrate of Nitrogen (FN2)=135.43kmol/hr X(0)=0 L(0)=0 Question: Solve dLdX=2FN2RNH3A using 4th order Runge Kutta method and plot a Conversion (X) vs Length (L) graph using Excel. Please show a detailed working of which values were used how to get the k1,k2k3 and k4 values using Length =1 as the example. Also provide Excel sheet if possible. Note: Please show a screenshot of the Excel sheet with the graph and estimate the length required at 97% conversion Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


