Answered step by step
Verified Expert Solution
Question
1 Approved Answer
CH10 (9) + 6.502 (9) 4CO2 (g) + 5H20 (v) an= -2658 kJ/mol 100 mol/s of n-butane at 300 C is burned with 50% excess
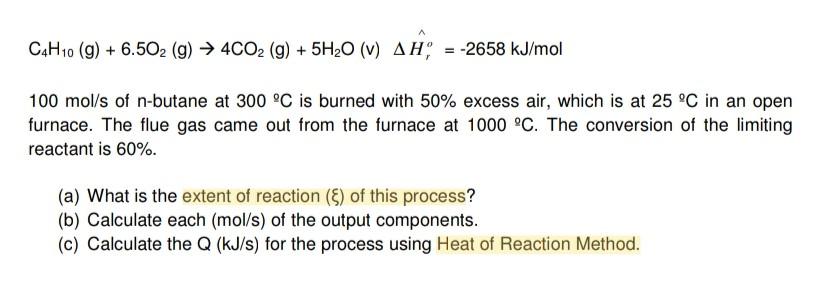
CH10 (9) + 6.502 (9) 4CO2 (g) + 5H20 (v) an= -2658 kJ/mol 100 mol/s of n-butane at 300 C is burned with 50% excess air, which is at 25C in an open furnace. The flue gas came out from the furnace at 1000 C. The conversion of the limiting reactant is 60%. (a) What is the extent of reaction () of this process? (b) Calculate each (mol/s) of the output components. (c) Calculate the Q (kJ/s) for the process using Heat of Reaction Method
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


