Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ch.5- 20, 23, 25 could you please help me get the correct answers i only have one try left 20 10 points Be sure to
Ch.5- 20, 23, 25
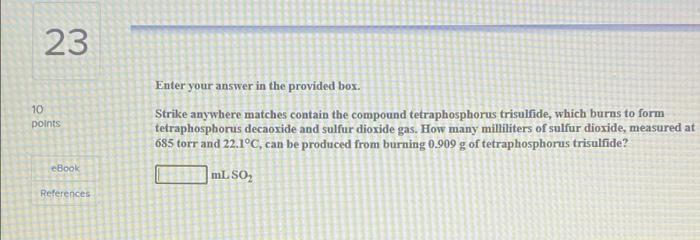
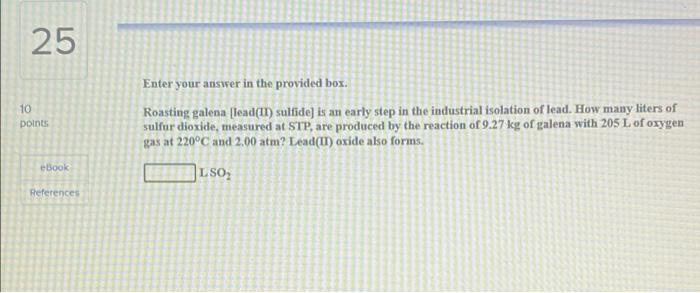
20 10 points Be sure to answer all parts. The air in a hot air balloon at 752 torr is heated from 23.0C to 51.0C. Assuming that the moles of air and the pressure remain constant, what is the density of the air at each temperature? (The average molar mass of air is 29.0 g/mol.) Density at 23.0C eBook References L Density at 51.0C 23 10 points Enter your answer in the provided box. Strike anywhere matches contain the compound tetraphosphorus trisulfide, which burns to form tetraphosphorus decaoxide and sulfur dioxide gas. How many milliliters of sulfur dioxide, measured at 685 torr and 22.1C, can be produced from burning 0.909 g of tetraphosphorus trisulfide? mL SOL ebook References 25 10 points Enter your answer in the provided box. Roasting galena (lead(II) sulfide) is an early step in the industrial isolation of lead. How many liters of sulfur dioxide, measured at STP, are produced by the reaction of 9.27 kg of galena with 205 L of oxygen gas at 220C and 2.00 atm? Lead(IT) oxide also forms. eBook LSO References could you please help me get the correct answers i only have one try left



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


