Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2) Changing dopant concentration in the Czochralski process 20 points total Since most impurities have a segregation coefficient smaller than 1 they enrich in

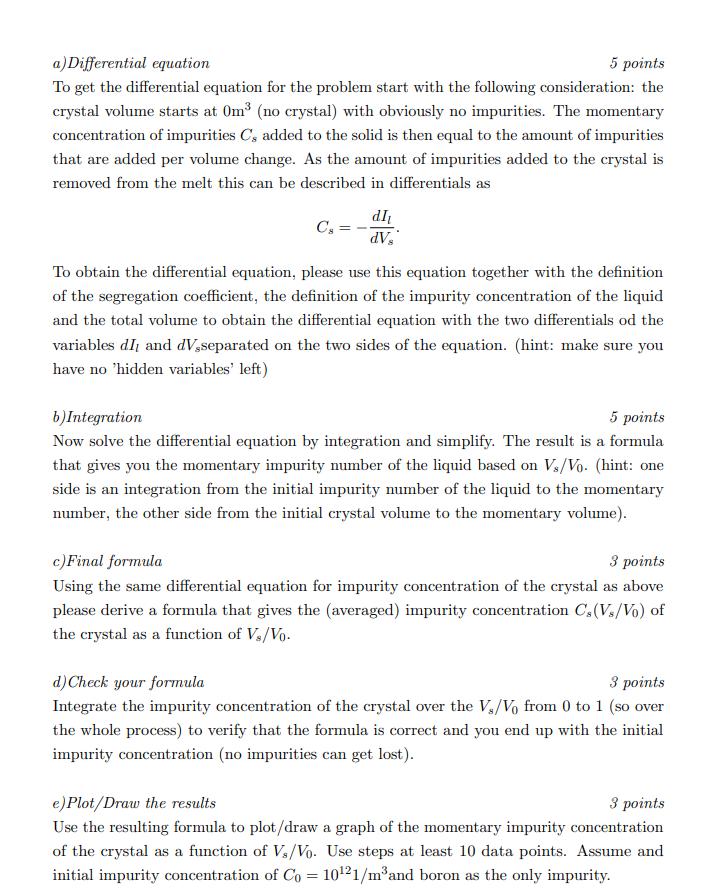

2) Changing dopant concentration in the Czochralski process 20 points total Since most impurities have a segregation coefficient smaller than 1 they enrich in the melt. However, the concentration of impurities in the melt directly influences the amount of impurities incorporated into the Si crystal in the Czochralski process. Thus, the resulting ingot will have an varying impurity concentration along its length. Here, we will derive a formula to obtain an estimation for the impurity concentration of the ingot depending on the volume fraction of the crystal at the moment the corresponding ingot section crystallized. As a first simplification assume that the density of the molten Si and the crystalline Si are identical. Therefore, the total volume (and thus the volume Vo of the melt at the start of the process) is simply the sum of the volume of the melt Vi and the volume of the crystal V, at any given time throughout the process. Please also note that the impurity concentration is the number of impurities per unit volume. The parameters used are the initial Volume Vo and impurity number Io of the melt. The variables are the momentary volume of the crystal V, and impurity number of the melt I at a given process state. The process state is defined by the fraction of solidified material Vs/Vo. a) Differential equation 5 points To get the differential equation for the problem start with the following consideration: the crystal volume starts at 0m (no crystal) with obviously no impurities. The momentary concentration of impurities C's added to the solid is then equal to the amount of impurities that are added per volume change. As the amount of impurities added to the crystal is removed from the melt this can be described in differentials as Cs= dI dV To obtain the differential equation, please use this equation together with the definition of the segregation coefficient, the definition of the impurity concentration of the liquid and the total volume to obtain the differential equation with the two differentials od the variables dI and dV,separated on the two sides of the equation. (hint: make sure you have no 'hidden variables' left) b) Integration 5 points Now solve the differential equation by integration and simplify. The result is a formula that gives you the momentary impurity number of the liquid based on Vs/Vo. (hint: one side is an integration from the initial impurity number of the liquid to the momentary number, the other side from the initial crystal volume to the momentary volume). c) Final formula 3 points Using the same differential equation for impurity concentration of the crystal as above please derive a formula that gives the (averaged) impurity concentration Cs (Vs/Vo) of the crystal as a function of V/Vo. d) Check your formula 3 points Integrate the impurity concentration of the crystal over the V/Vo from 0 to 1 (so over the whole process) to verify that the formula is correct and you end up with the initial impurity concentration (no impurities can get lost). e) Plot/Draw the results 3 points Use the resulting formula to plot/draw a graph of the momentary impurity concentration of the crystal as a function of Vs/Vo. Use steps at least 10 data points. Assume and initial impurity concentration of Co= 1021/m and boron as the only impurity. f)Stop condition 1 point At which point of this process would you need to stop it if you wanted to have a maximum impurity concentration Cmax = 1021/m in any part of the ingot? (Same assumptions as in the previous task)
Step by Step Solution
★★★★★
3.59 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


