Answered step by step
Verified Expert Solution
Question
1 Approved Answer
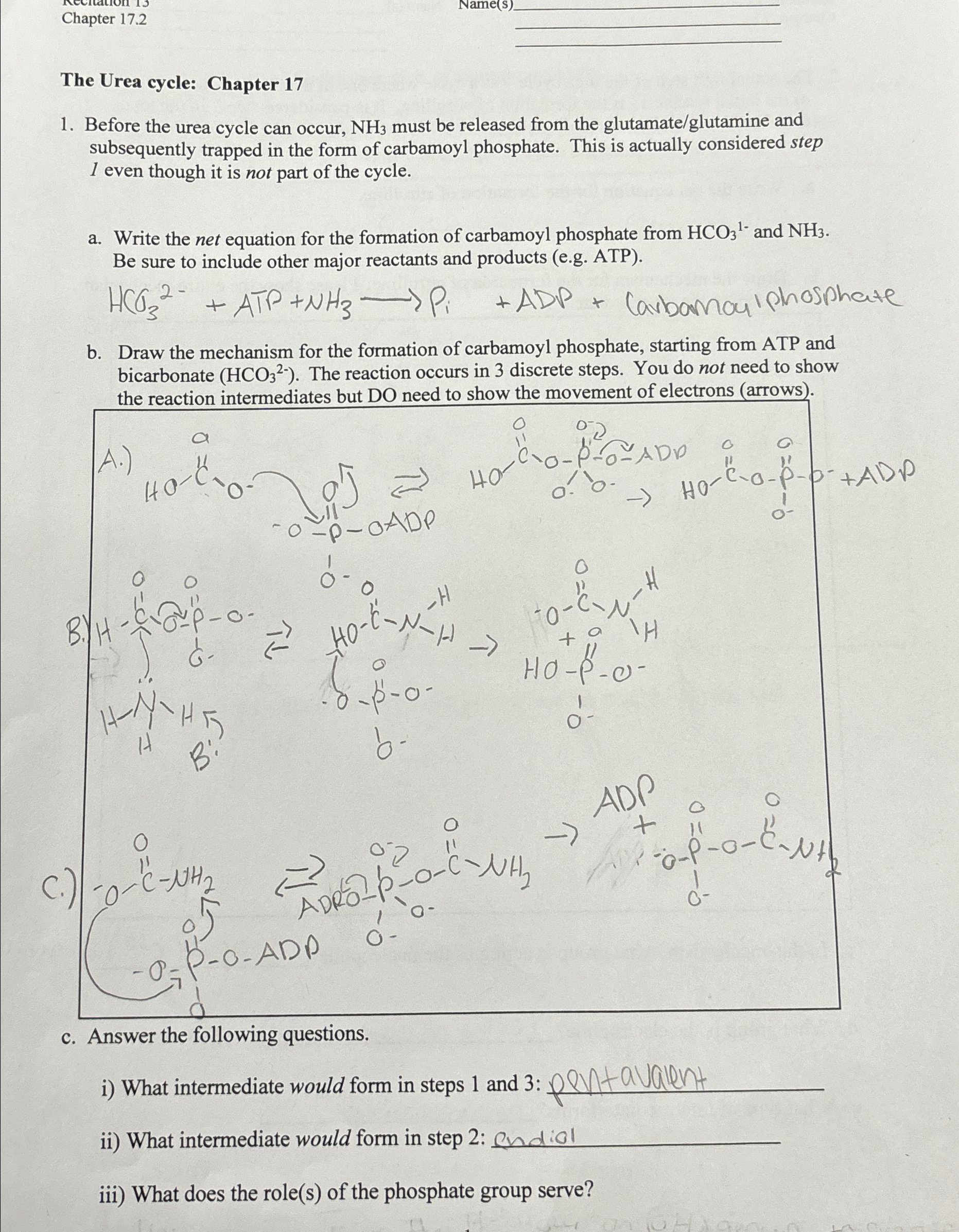
Chapter 1 7 . 2 The Urea cycle: Chapter 1 7 Before the urea cycle can occur, N H 3 must be released from the
Chapter
The Urea cycle: Chapter
Before the urea cycle can occur, must be released from the glutamateglutamine and subsequently trapped in the form of carbamoyl phosphate. This is actually considered step even though it is not part of the cycle.
a Write the net equation for the formation of carbamoyl phosphate from and Be sure to include other major reactants and products eg ATP
ATPADP Carbamouiphosphate
b Draw the mechanism for the formation of carbamoyl phosphate, starting from ATP and bicarbonate The reaction occurs in discrete steps. You do not need to show the reaction intermediates but DO need to show the movement of electrons arrows
c Answer the following questions.
i What intermediate would form in steps and : pentavalent
ii What intermediate would form in step : endiol
iii What does the roles of the phosphate group serve?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


