Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chapter 7: Homework 2 Chapter 7: Homework 2 Question 1 of 10 < > View Policies Current Attempt in Progress -/10 In a certain
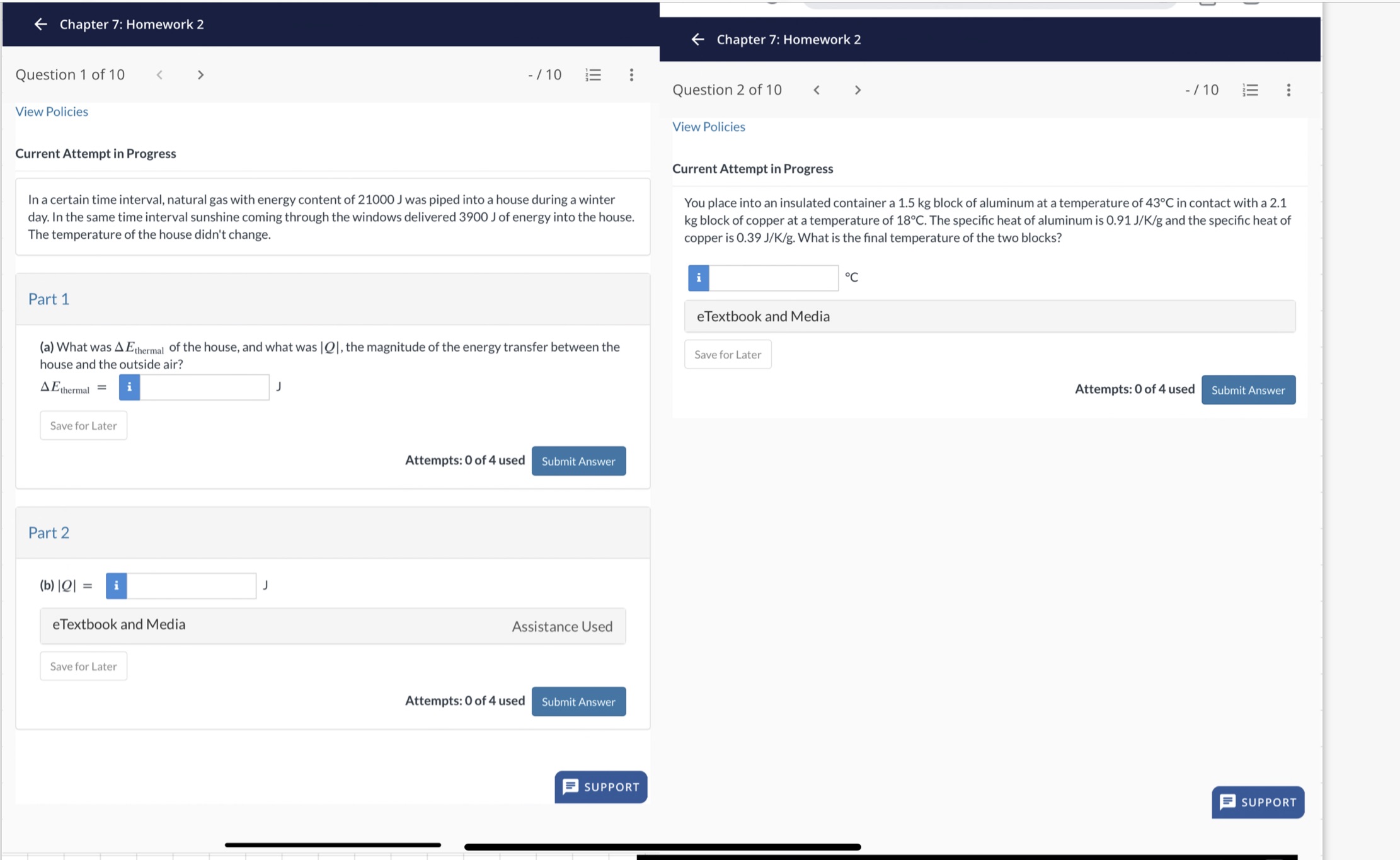
Chapter 7: Homework 2 Chapter 7: Homework 2 Question 1 of 10 < > View Policies Current Attempt in Progress -/10 In a certain time interval, natural gas with energy content of 21000 J was piped into a house during a winter day. In the same time interval sunshine coming through the windows delivered 3900 J of energy into the house. The temperature of the house didn't change. Question 2 of 10 < > View Policies Current Attempt in Progress -/10 E You place into an insulated container a 1.5 kg block of aluminum at a temperature of 43C in contact with a 2.1 kg block of copper at a temperature of 18C. The specific heat of aluminum is 0.91 J/K/g and the specific heat of copper is 0.39 J/K/g. What is the final temperature of the two blocks? Part 1 (a) What was AEthermal of the house, and what was |Q|, the magnitude of the energy transfer between the house and the outside air? AE thermal = i J Save for Later Part 2 (b)|Q| eTextbook and Media Save for Later Attempts: 0 of 4 used Submit Answer Assistance Used Attempts: 0 of 4 used Submit Answer eTextbook and Media Save for Later C Attempts: 0 of 4 used Submit Answer SUPPORT SUPPORT
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


