Answered step by step
Verified Expert Solution
Question
1 Approved Answer
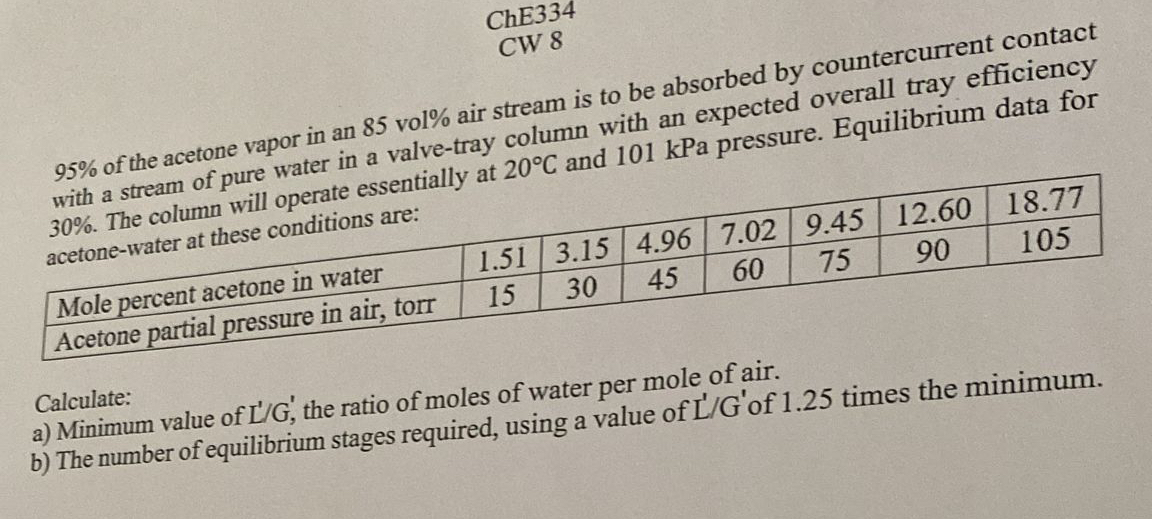
ChE 3 3 4 CW 8 9 5 % of the acetone vapor in an 8 5 vol % air stream is to be absorbed
ChE
CW
of the acetone vapor in an vol air stream is to be absorbed by countercurrent contact with a stream of pure water in a valvetray column with an expected overall tray efficiency The column will operate essentially at and kPa pressure. Equilibrium data for acetonewater at these conditions are:
tableMole percent acetone in water,Acetone partial pressure in air, torr,
Calculate:
a Minimum value of the ratio of moles of water per mole of air.
b The number of equilibrium stages required, using a value of of times the minimum.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


