Question
Deduce the structure of this compound using the spectroscopic information. Assign all significant peaks in all spectra. Chem 2125 Lab MS: T mass spectrum of
Deduce the structure of this compound using the spectroscopic information. Assign all significant peaks in all spectra. 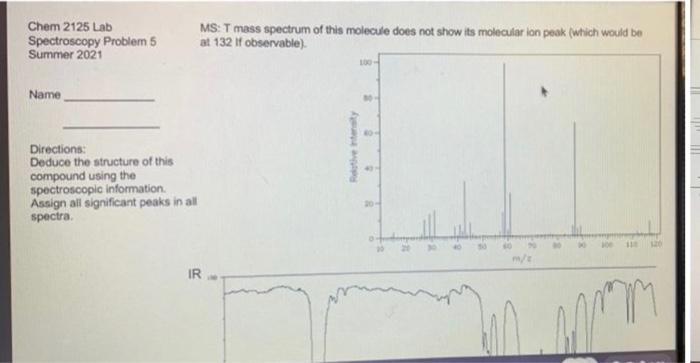
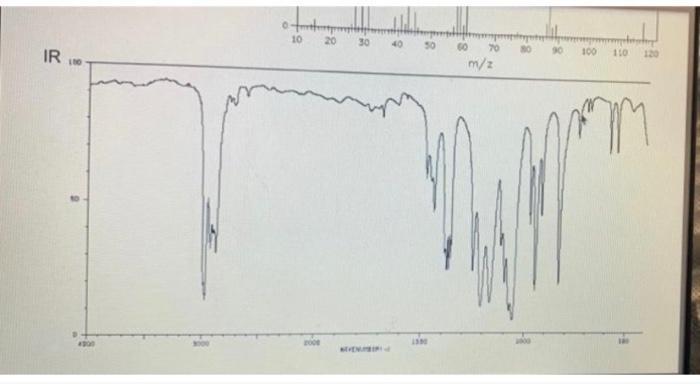
Chem 2125 Lab MS: T mass spectrum of this molecule does not show its molecular ion peak (which would be at 132 if observable). Spectroscopy Problem 5 Summer 2021 Name Directions: Deduce the structure of this compound using the spectroscopic information. Assign all significant peaks in all spectra. 20- m/e IR Retive intensity
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
From the IR spectra you can see a peak at around 1715 cm1 this peak corresponds to a carbony...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Francis A. Carey
4th edition
0072905018, 978-0072905014
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



