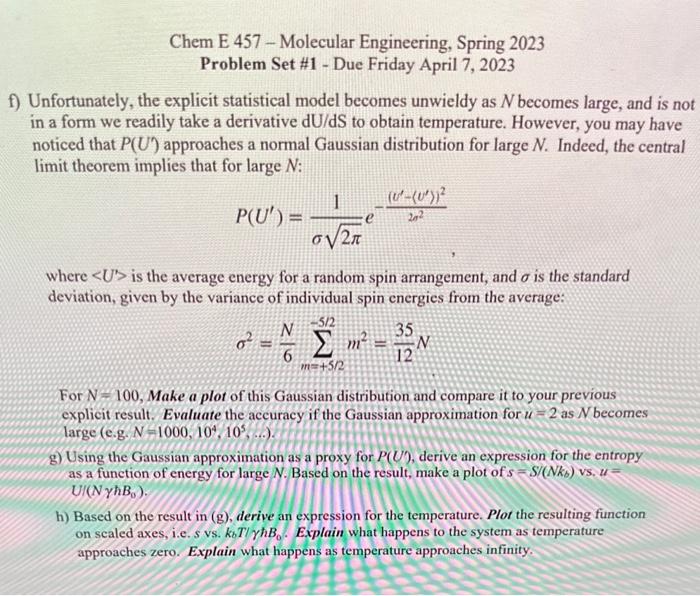
Chem E 457 - Molecular Engineering, Spring 2023 Problem Set \#1 - Due Friday April 7, 2023 Unfortunately, the explicit statistical model becomes unwieldy as N becomes large, and is not in a form we readily take a derivative dU/dS to obtain temperature. However, you may have noticed that P(U) approaches a normal Gaussian distribution for large N. Indeed, the central limit theorem implies that for large N : P(U)=21e22(U(U))2 where U is the average energy for a random spin arrangement, and is the standard deviation, given by the variance of individual spin energies from the average: 2=6Nm=+5/25/2m2=1235N For N=100, Make a plot of this Gaussian distribution and compare it to your previous explicit result. Evaluate the accuracy if the Gaussian approximation for u=2 as N becomes large (e.g. N=1000,104,105, ) g) Using the Gaussian approximation as a proxy for P(U), derive an expression for the entropy as a function of energy for large N. Based on the result, make a plot of s=S/(Nkb) vs, u= h) Based on the result in (g), derive an expression for the temperature. Plot the resulting function on scaled axes, i.e. s vs. kbT/B0. Explain what happens to the system as temperature approaches zero. Explain what happens as temperature approaches infinity. Chem E 457 - Molecular Engineering, Spring 2023 Problem Set \#1 - Due Friday April 7, 2023 Unfortunately, the explicit statistical model becomes unwieldy as N becomes large, and is not in a form we readily take a derivative dU/dS to obtain temperature. However, you may have noticed that P(U) approaches a normal Gaussian distribution for large N. Indeed, the central limit theorem implies that for large N : P(U)=21e22(U(U))2 where U is the average energy for a random spin arrangement, and is the standard deviation, given by the variance of individual spin energies from the average: 2=6Nm=+5/25/2m2=1235N For N=100, Make a plot of this Gaussian distribution and compare it to your previous explicit result. Evaluate the accuracy if the Gaussian approximation for u=2 as N becomes large (e.g. N=1000,104,105, ) g) Using the Gaussian approximation as a proxy for P(U), derive an expression for the entropy as a function of energy for large N. Based on the result, make a plot of s=S/(Nkb) vs, u= h) Based on the result in (g), derive an expression for the temperature. Plot the resulting function on scaled axes, i.e. s vs. kbT/B0. Explain what happens to the system as temperature approaches zero. Explain what happens as temperature approaches infinity