Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ChemActivity 1 Inebule ertOT The Nuclear Atom (What Is an Atom?) Model: Schematic Diagrams for Various Atoms and lons. electron (-) o proton (+)
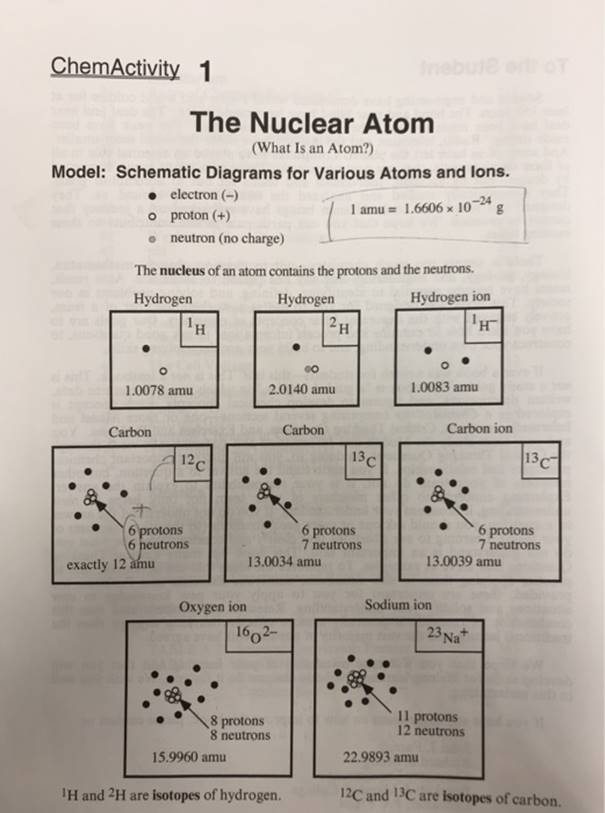
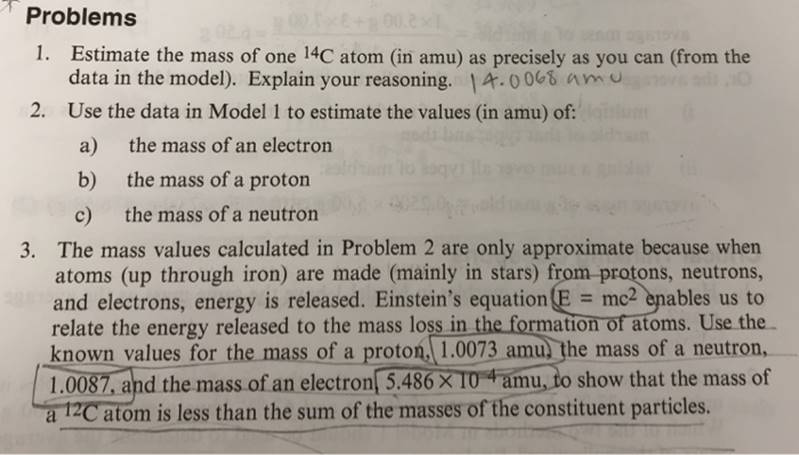
ChemActivity 1 Inebule ertOT The Nuclear Atom (What Is an Atom?) Model: Schematic Diagrams for Various Atoms and lons. electron (-) o proton (+) I amu = 1.6606 x 102 g -24 %3D neutron (no charge) The nucleus of an atom contains the protons and the neutrons. Hydrogen Hydrogen Hydrogen ion TH 2H 1.0078 amu 2.0140 amu 1.0083 amu Carbon ion Carbon 12c Carbon 13C 13C 6 protons 6 neutrons 6 protons 7 neutrons 6 protons 7 neutrons 13.0039 amu exactly 12 amu 13.0034 amu Oxygen ion Sodium ion 1602- 23 Na+ 8 protons 8 neutrons 11 protons 12 neutrons 15.9960 amu 22.9893 amu IH and 2H are isotopes of hydrogen. 12C and 13C are isotopes of carbon. Problems 1. Estimate the mass of one 14C atom (in amu) as precisely as you can (from the data in the model). Explain your reasoning. 4.0068 amu 2. Use the data in Model 1 to estimate the values (in amu) of: a) the mass of an electron b) the mass of a proton c) the mass of a neutron 3. The mass values calculated in Problem 2 are only approximate because when atoms (up through iron) are made (mainly in stars) from protons, neutrons, and electrons, energy is released. Einstein's equation(E mc2 enables us to relate the energy released to the mass loss in the formation of atoms. Use the. known values for the mass of a proton, 1.0073 amu) the mass of a neutron, 1.0087, and the mass of an electron, 5.486 X 10 amu, to show that the mass of 12C atom is less than the sum of the masses of the constituent particles. a
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
1 The mass of one 14C atom is estimated to be 140068 amu This value is obtained by adding the masses ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


