Answered step by step
Verified Expert Solution
Question
1 Approved Answer
chemical kinetics please help 6) For the following reaction at 600KJmol1 having 323KJmol1 as dissociation energy PCl5PCl3+Cl2 The RRK theory predicted rate, given the high
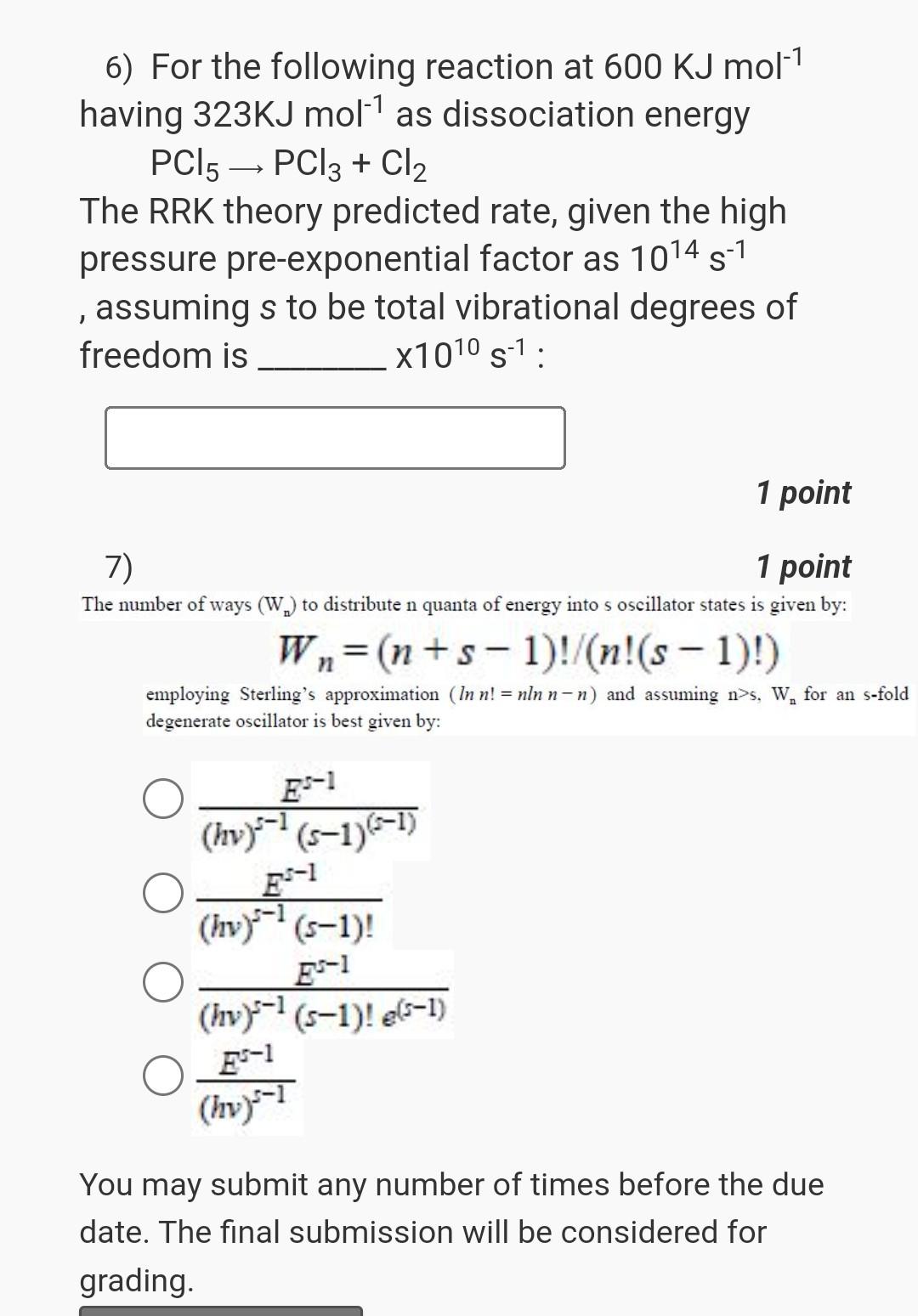

chemical kinetics please help
6) For the following reaction at 600KJmol1 having 323KJmol1 as dissociation energy PCl5PCl3+Cl2 The RRK theory predicted rate, given the high pressure pre-exponential factor as 1014s1 , assuming s to be total vibrational degrees of freedom is 1010s1 : 1 point 7) 1 point The number of ways (Wn) to distribute n quanta of energy into s oscillator states is given by: Wn=(n+s1)!/(n!(s1)!) employing Sterling's approximation (lnn!=nlnnn) and assuming n>s,Wn for an s-fold degenerate oscillator is best given by: (hv)s1(s1)(s1)Es1(hv)s1(s1)!Es1(hv)s1(s1)!e(s1)Es1(hv)s1Es1 You may submit any number of times before the due date. The final submission will be considered for grading. 7) The number of ways (Wn) to distribute n quanta of energy into s oscillator states is given by: 1 poin Wn=(n+s1)!/(n!(s1)!) employing Sterling's approximation (lnn!=nlnnn ) and assuming n>s,Wn for an s-fold degenerate oscillator is best given by: (hv)s1(s1)(s1)Es1(hv)s1(s1)!Es1(hv)s1(s1)!e(s1)Es1(hv)s1Es1 You may submit any number of times before the due date. The final submission will be considered for gradingStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


