Question
- Chemical Reaction Engineering problem - If you need to draw a graph when solving a problem, use the Polymath program to draw a graph
- Chemical Reaction Engineering problem -
If you need to draw a graph when solving a problem, use the Polymath program to draw a graph
The following reactions are carried out isothermally in a 50 dm^3 PFR :
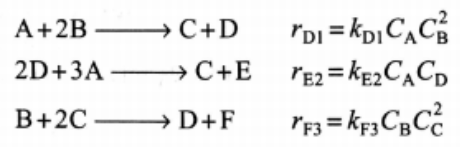
Additional information : Liquid phase

( c ) plot the species concentrations and the conversion of A as a function of time when the reaction is carried out in a semibatch reactor initially containing 40 dm^3 of liquid. Consider two cases (1) A is fed to B, and (2) B is fed to A. What differences do you observe for these two cases?
If you need to draw a graph when solving a problem, use the Polymath program to draw a graph
Please write neatly so that I can read it easily
A+2BC+D2D+3AC+EB+2CD+FrD1=kD1CACB2rE2=kE2CACDrF3=kF3CBCC2 kD1kE2=0.1dm3/molminkF3=5.0dm6/mol2min=0.25dm6/mol2minCA0=1.5mol/dm3CB0=2.0mol/dm3v0=10dm3/minStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


