Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical Reaction Engineering -Show each step clearly in your derivations required in the solution of each problem. - DO NOT USE the final equations directly
Chemical Reaction Engineering
-Show each step clearly in your derivations required in the solution of each problem.
- DO NOT USE the final equations directly from your textbook.
- R: 1.987cal/molK, 0.082 L.atm/mol.K, 8.314 J/mol.K
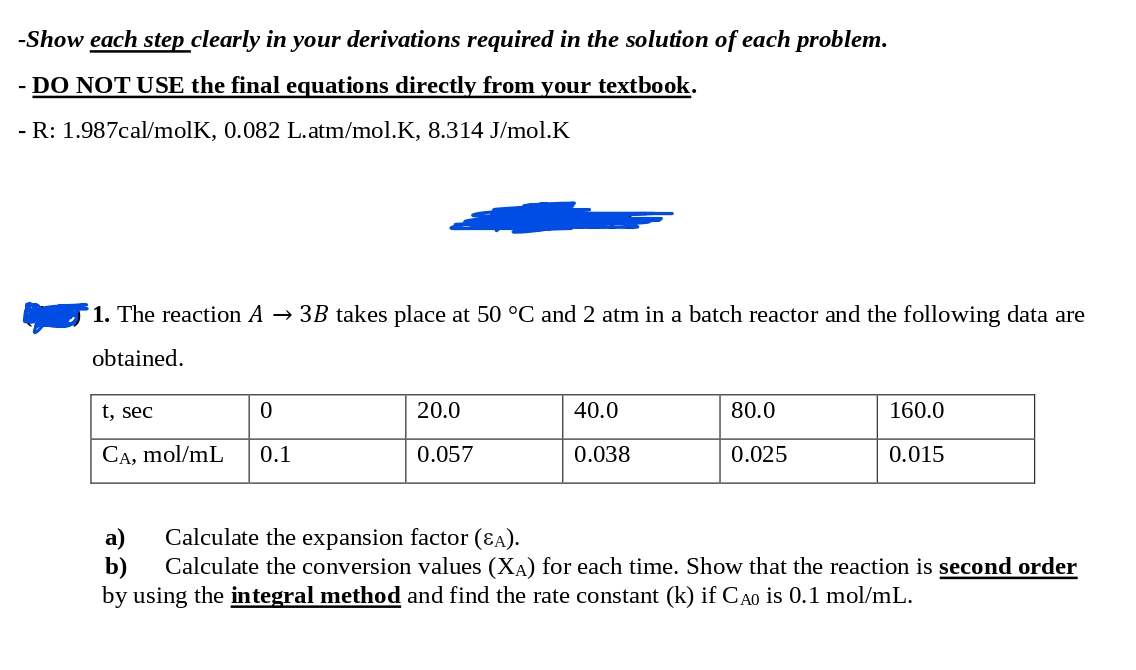 -Show each step clearly in your derivations required in the solution of each problem. - DO NOT USE the final equations directly from your textbook. - R: 1.987cal/molK, 0.082L.atm/mol.K,8.314J/mol.K 1. The reaction A3B takes place at 50C and 2atm in a batch reactor and the following data are obtained. a) Calculate the expansion factor (A). b) Calculate the conversion values (XA) for each time. Show that the reaction is second order by using the integral method and find the rate constant (k) if CA0 is 0.1mol/mL
-Show each step clearly in your derivations required in the solution of each problem. - DO NOT USE the final equations directly from your textbook. - R: 1.987cal/molK, 0.082L.atm/mol.K,8.314J/mol.K 1. The reaction A3B takes place at 50C and 2atm in a batch reactor and the following data are obtained. a) Calculate the expansion factor (A). b) Calculate the conversion values (XA) for each time. Show that the reaction is second order by using the integral method and find the rate constant (k) if CA0 is 0.1mol/mL Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


