Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical reactions in foods that follow zero-order kinetics exhibit a constant rate of change in the concentration of a reactant or a product. When
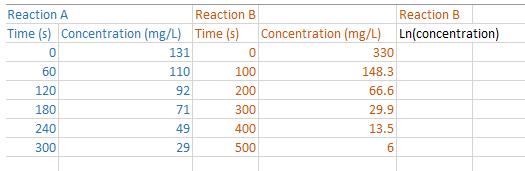
Chemical reactions in foods that follow zero-order kinetics exhibit a constant rate of change in the concentration of a reactant or a product. When the concentration is plotted against time, a straight line graph is obtained. The slope of the straight line gives the zero-order rate constant. On the other hand, many chemical reactions that occur during food processing and storage follow first-order kinetics. If we plot the concentration of a reactant against time we get an exponential type plot (i.e., a curve). The rate constant for a first- order reaction can be obtained by taking the natural logarithm of concentrations and then plotting those values against time. If the reaction follows first-order kinetics, then a plot of the natural logarithm of concentration against time should yield a straight line. Two sets of data are shown in the table below. a) Obtain a scatterplot of Concentration vs Time for Reaction A. (Be sure to put time on the x-axis). What trend do you see? Obtain a correlation coefficient. What does it tell you? What order of kinetics is being shown by Reaction A? b) Obtain a scatterplot of Concentration vs Time for Reaction B. (Be sure to put time on the x-axis). What trend do you see? What order of kinetics is being shown by Reaction B? c) Transform the concertation data for Reaction B by taking the natural logarithm. Obtain a scatterplot of Ln Concentration vs Time. What trend do you see now? d) Obtain a correlation coefficient for the relationship between Ln Concentration and Time. What does it tell you? Reaction A Reaction B Time (s) Concentration (mg/L) Time (s) 0 60 120 180 240 300 131 110 92 71 49 29 0 100 200 300 400 500 Concentration (mg/L) 330 148.3 66.6 29.9 13.5 6 Reaction B Ln(concentration)
Step by Step Solution
★★★★★
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
A Obtain a scatterplot of Concentration vs Time for Reaction A Be sure to put time on the xaxis What trend do you see Obtain a correlation coefficient ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started