Answered step by step
Verified Expert Solution
Question
1 Approved Answer
. Chemistry 11201 Name: CH-CH-CH-CH, CH, b.p. - 27.9C [fuels and fuel additives] CH, b.p. - 9.5C [petroleum naphtha] Alkenes Ethene (ethylene) --103.7C 2,2,4-trimethyl-
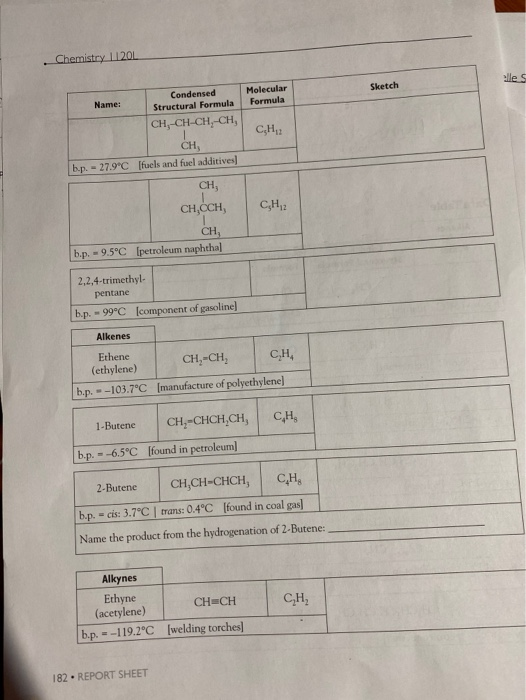
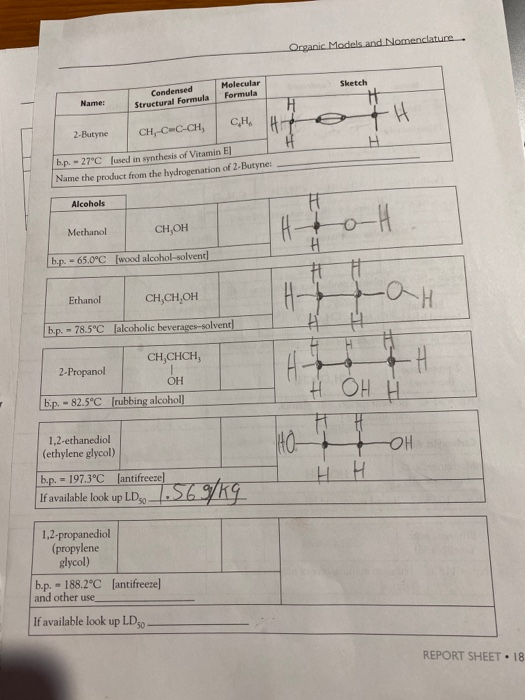
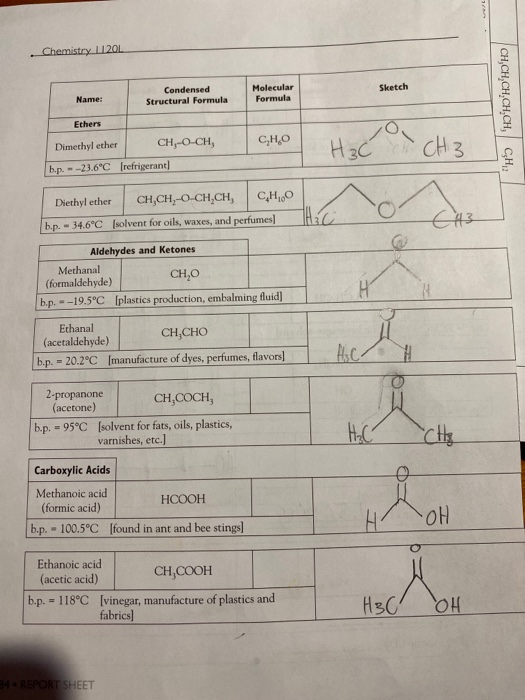

. Chemistry 11201 Name: CH-CH-CH-CH, CH, b.p. - 27.9C [fuels and fuel additives] CH, b.p. - 9.5C [petroleum naphtha] Alkenes Ethene (ethylene) --103.7C 2,2,4-trimethyl- pentane b.p. - 99C [component of gasoline] b.p. - 1-Butene --6.5C Condensed Structural Formula b.p. = CH, CH,CCH, Alkynes Ethyne (acetylene) b.p.=-119.2C 182 REPORT SHEET [found in petroleum] Molecular Formula CH2 CH=CH CH [manufacture of polyethylene] CH-CHCH,CH, CH2 2-Butene CH,CH-CHCH, CH b.p. - cis: 3.7C | trans: 0.4C [found in coal gas] Name the product from the hydrogenation of 2-Butene: CH=CH [welding torches] CH CH Sketch elle S Name: Alcohols 2-Butyne CH-C-C-CH, b.p. -27C [used in synthesis of Vitamin EJ Name the product from the hydrogenation of 2-Butyne: Methanol CH,OH b.p. - 65.0C [wood alcohol-solvent] Ethanol Condensed Structural Formula 2-Propanol b.p. -78.5C [alcoholic beverages-solvent] CH,CHCH, OH b.p. -82.5C [rubbing alcohol] 1,2-ethanediol (ethylene glycol) CH,CH,OH 1,2-propanediol (propylene glycol) Molecular Formula CH b.p. = 197.3C [antifreeze] If available look up LD 569/kg - b.p. 188.2C [antifreeze] and other use, If available look up LD 50- Organic Models and Nomenclature. H == H- HO- H Sketch H +H H H H H OH H H H H H OH H REPORT SHEET 18 Chemistry 11201 Name: Ethers Dimethyl ether b.p.--23.6C [refrigerant] Ethanal (acetaldehyde) 2-propanone (acetone) Diethyl ether CH,CH,O CHCH, CH0O b.p. - 34.6C [solvent for oils, waxes, and perfumes] Aldehydes and Ketones Methanal CHO (formaldehyde) b.p.-19.5C [plastics production, embalming fluid] Condensed Structural Formula Carboxylic Acids Methanoic acid (formic acid) - CH, Q.CH, CH,CHO b.p. 20.2C [manufacture of dyes, perfumes, flavors] = CH,COCH, b.p. 95C [solvent for fats, oils, plastics, varnishes, etc.] HCOOH b.p. 100.5C [found in ant and bee stings] Ethanoic acid (acetic acid) Molecular Formula 34 REPORT SHEET CHO CHCOOH b.p. 118C [vinegar, manufacture of plastics and fabrics] H3C HC 10 H HC- Sketch HC H H. CH 3 H3C C113 OH Mon OH CHS CH,CH,CH,CH,CH, CH2 3: Ath focht Name: Amines Condensed Structural Formula Aminomethane (methylamine) b.p.-6.3C Jused in tanningl CHNH, 1,4-diaminobutane NH,CH,CH,CH,CHNH, (putrescine) Molecular Formula b.p. 158C [found in cells, used in biochemical research] Organic Models and Nomenclature. Sketch Observations of Demonstrations 1. Combustion of alcohols. Observe the color and characteristics of the flames: a. Methanol (CH,OH) b. Ethanol (CH,CH,OH) c. 2-propanol (CH,CHCH) OH What trend is observed as the number of carbons in the chain increases? Do you think that it is possible that products other than carbon dioxide and water vapor are pro- duced when these substances burn? What would make you suspect this? CH,CHOH(1) + 3 O(g)-2 CO(g) + 3 HO(g) (Combustion of ethanol) 2. Bromination of vegetable oil. What happens when the vegetable oil is added to the test tube containing the bromine? REPORT SHEET 185
Step by Step Solution
★★★★★
3.40 Rating (141 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


