Question
6. Consider the following concentration table. 0(8) 2.0 mol I (initial moles) C (change) E (equilibrium moles) 3 H(g) + 4.0 mol 2 NH3(g)
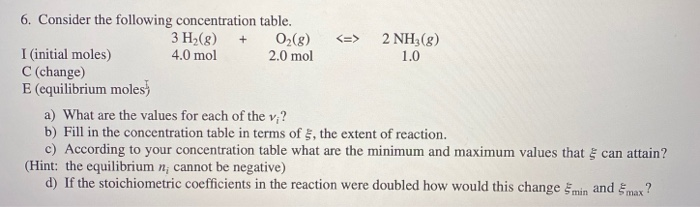
6. Consider the following concentration table. 0(8) 2.0 mol I (initial moles) C (change) E (equilibrium moles) 3 H(g) + 4.0 mol 2 NH3(g) 1.0 a) What are the values for each of the vi? b) Fill in the concentration table in terms of 5, the extent of reaction. c) According to your concentration table what are the minimum and maximum values that can attain? (Hint: the equilibrium n; cannot be negative) d) If the stoichiometric coefficients in the reaction were doubled how would this change min and max?
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Applied Electromagnetics
Authors: Fawwaz T. Ulaby, Eric Michielssen, Umberto Ravaioli
6th edition
132139316, 978-0132139311
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



