Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4) Use Table 2.3 from your text book to determine the physical state (solid, liquid, or gas) of the following chemicals: Ethane, Ethanol, Hexane,

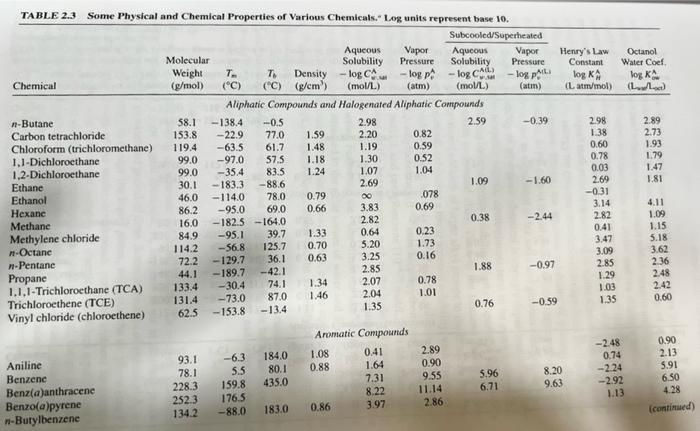
4) Use Table 2.3 from your text book to determine the physical state (solid, liquid, or gas) of the following chemicals: Ethane, Ethanol, Hexane, Methane, Methanol, and Naphthalene. Note: Just do not state your answer, show how you determined the physical state of each of these pollutants using relevant physical properties listed in Table 2.3. TABLE 2.3 Some Physical and Chemical Properties of Various Chemicals. Log units represent base 10. Subcooled/Superheated Chemical n-Butane Carbon tetrachloride Chloroform (trichloromethane) 1,1-Dichloroethane 1,2-Dichloroethane Ethane Ethanol Hexane Methane Methylene chloride n-Octane n-Pentane Propane 1.1.1-Trichloroethane (TCA) Trichloroethene (TCE) Vinyl chloride (chloroethene) Aniline Benzene Benz(a)anthracene Benzo(a)pyrene n-Butylbenzene Molecular Weight T To Density (g/mol) (C) (C) (g/cm) 58.1 153.8 119.4 99.0 99.0 Aqueous Vapor Solubility Pressure -log CA -log p (atm) F (mol/L) Aliphatic Compounds and Halogenated Aliphatic Compounds 2.98 2.59 2.20 1.19 1.30 1.07 2.69 30.1 46.0 86.2 16.0 84.9 114.2 72.2 -138.4 -0.5 -22.9 77.0 61.7 -63.5 -97.0 -35.4 -183.3 -114.0 -95.0 -182.5 -164.0 -95.1 -56.8 -129.7 44.1 -189.7 -42.1 133.4 -30.4. 131.4 62.5 57.5 83.5 -88.6 78.0 0.79 69.0 0.66 93.1 -6.3 78.1 5.5 228.3 159.8 252.3 176.5 134.2 -88.0 39.7 125.7 36.1 74.1 -73.0 87.0 -153.8 -13.4 184.0 80.1 435.0 1.59 1.48 1.18 1.24 183.0 1.33 0.70 0.63 1.34 1.46 1.08 0.88 0 3.83 2.82 0.64 5.20 3.25 2.85 Aromatic Compounds 0.41 1.64 7.31 8.22 3.97 0.86 2.07 2.04 1.35 0.82 0.59 0.52 1.04 078 0.69 0.23 1.73 0.16 0.78 1.01 2.89 0.90 9.55 11.14 2.86 Aqueous Vapor Solubility Pressure -log CA -log p (mol/L) 1.09 0.38 1.88 0.76 5.96 6.71 (atm) -0.39 -1.60 -2.44 -0.97 -0.59 Henry's Law Constant log KA (L atm/mol) 8.20 9.63 2.98 1.38 0.60 0.78 0.03 2.69 -0.31 3.14 2.82 0.41 3.47 3.09 2.85 1.29 1.03 1.35 Octanol Water Coef. log K (L) -2.48 0.74 -2.24 -2.92 1.13 2.89 2.73 1.93 1.79 1.47 1.81 4.11 1.09 1.15 5.18 3.62 2.36 2.48 2.42 0.60 0.90 2.13 5.91 6.50 4.28 (continued)
Step by Step Solution
★★★★★
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
To solve this question we need to first knowing about the metting point and boiling point of any s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


