Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemistry CH + H C6H6 + CH4 C6H6+C6H6 C2H10 + H 25E09 pound/year market at $6.50/ gallon Benzene is a suspected carcinogen 550C, 55
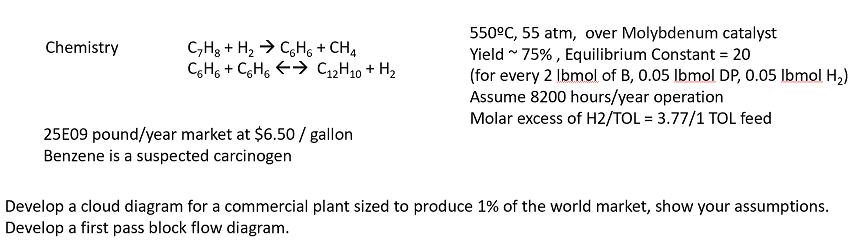
Chemistry CH + H C6H6 + CH4 C6H6+C6H6 C2H10 + H 25E09 pound/year market at $6.50/ gallon Benzene is a suspected carcinogen 550C, 55 atm, over Molybdenum catalyst Yield ~ 75%, Equilibrium Constant = 20 (for every 2 lbmol of B, 0.05 lbmol DP, 0.05 lbmol H) Assume 8200 hours/year operation Molar excess of H2/TOL = 3.77/1 TOL feed Develop a cloud diagram for a commercial plant sized to produce 1% of the world market, show your assumptions. Develop a first pass block flow diagram.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To develop a cloud diagram and a first pass block flow diagram for a commercial plant to produce ben...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


