Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Trial 1 Trial 2 Trail 3 Average Metal Pairs Anode Cathode Zinc Copper Calculations: Cu/C -0.321 -0.347 -0.262 -0.310 V Pb/C -0.737 -0.745 -0.762
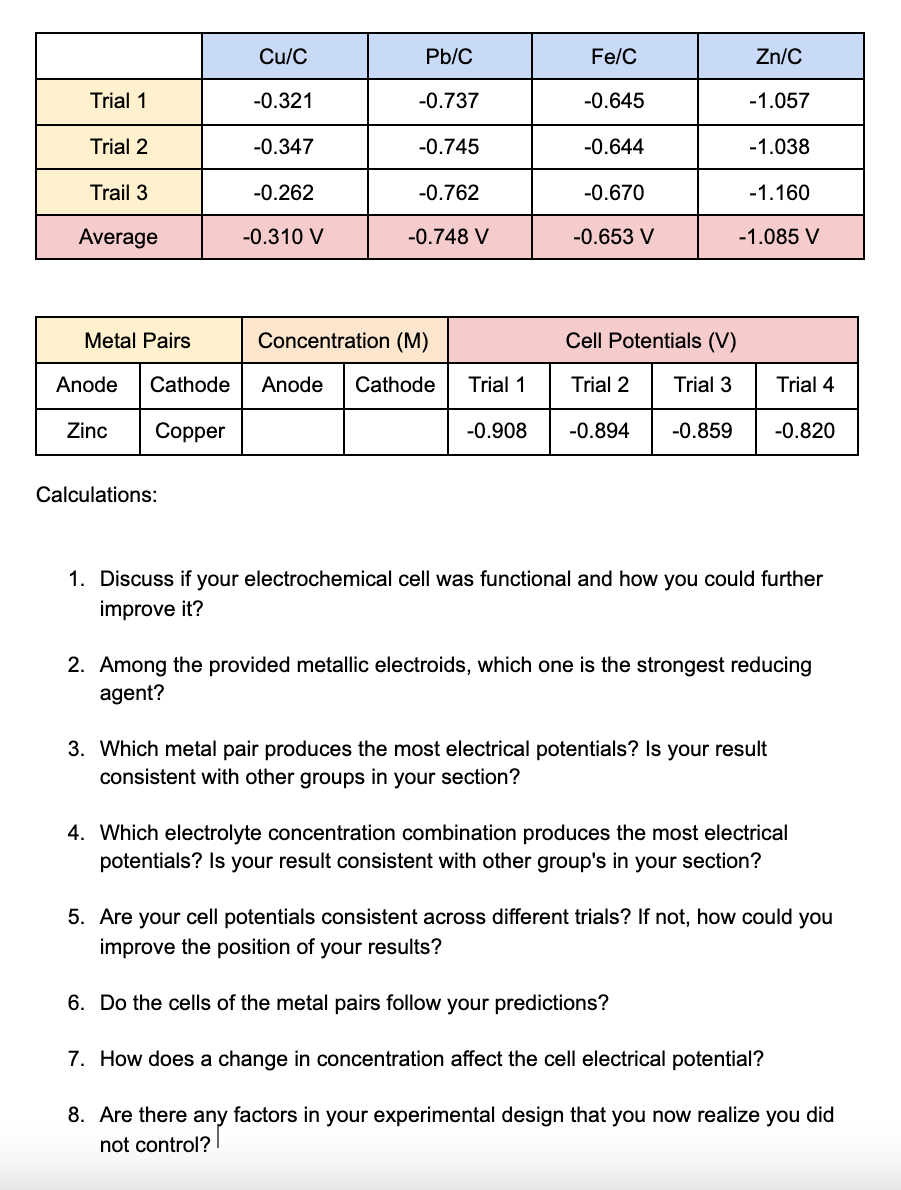
Trial 1 Trial 2 Trail 3 Average Metal Pairs Anode Cathode Zinc Copper Calculations: Cu/C -0.321 -0.347 -0.262 -0.310 V Pb/C -0.737 -0.745 -0.762 -0.748 V Concentration (M) Anode Cathode Fe/C -0.645 -0.644 -0.670 -0.653 V Cell Potentials (V) Trial 1 Trial 2 Trial 3 -0.908 -0.894 -0.859 Zn/C -1.057 -1.038 -1.160 -1.085 V Trial 4 -0.820 1. Discuss if your electrochemical cell was functional and how you could further improve it? 3. Which metal pair produces the most electrical potentials? Is your result consistent with other groups in your section? 2. Among the provided metallic electroids, which one is the strongest reducing agent? 4. Which electrolyte concentration combination produces the most electrical potentials? Is your result consistent with other group's in your section? 5. Are your cell potentials consistent across different trials? If not, how could you improve the position of your results? 6. Do the cells of the metal pairs follow your predictions? 7. How does a change in concentration affect the cell electrical potential? 8. Are there any factors in your experimental design that you now realize you did not control?
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
1 Discuss if your electrochemical cell was functional and how you could further improve it The electrochemical cell we used was functional We were able to measure the potentials of the metals we used ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


