Question
The molarity of the Cu+ and Zn+ solutions used in the setup of the electrochemical cell was 1 M. Explain why the voltage was
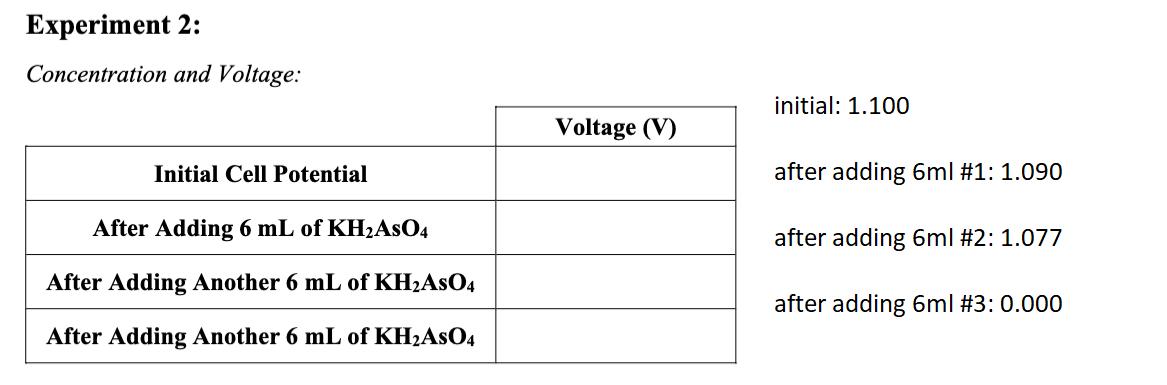
The molarity of the Cu+ and Zn+ solutions used in the setup of the electrochemical cell was 1 M. Explain why the voltage was not equal to the standard cell potential for the Cu/Zn redox reaction at all times during the experiment. Experiment 2: Concentration and Voltage: Initial Cell Potential After Adding 6 mL of KHAsO4 After Adding Another 6 mL of KHASO4 After Adding Another 6 mL of KHASO4 Voltage (V) initial: 1.100 after adding 6ml #1: 1.090 after adding 6ml #2: 1.077 after adding 6ml #3: 0.000
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Linear Algebra
Authors: Peter J. Olver, Cheri Shakiban
1st edition
131473824, 978-0131473829
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App