Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(e) In a vanadium flow battery, the electrochemical reactions which take place at the electrodes during discharge are: At the positive electrode: (VO2)2SO4 +
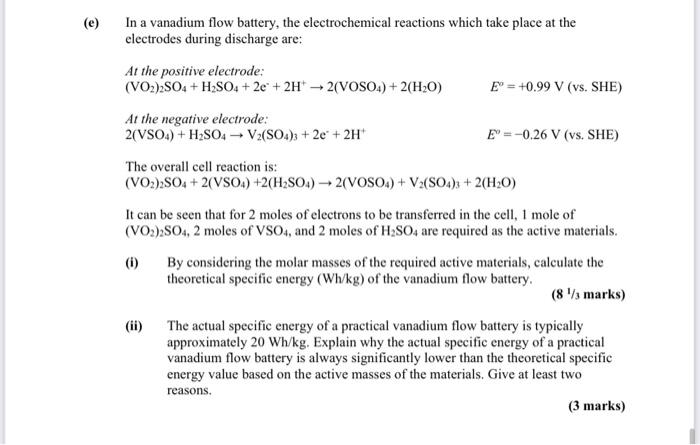
(e) In a vanadium flow battery, the electrochemical reactions which take place at the electrodes during discharge are: At the positive electrode: (VO2)2SO4 + HSO4 +2e + 2H2(VOSO4) + 2(HO) At the negative electrode: 2(VSO4) + HSO4 V2(SO4)3 +2e + 2H+ E = +0.99 V (vs. SHE) The overall cell reaction is: (VO2)2SO4 + 2(VSO4) +2(HSO4)2(VOSO4) + V2(SO4)3 + 2(HO) (i) E-0.26 V (vs. SHE) It can be seen that for 2 moles of electrons to be transferred in the cell, 1 mole of (VO2)2SO4, 2 moles of VSO4, and 2 moles of HSO4 are required as the active materials. (ii) By considering the molar masses of the required active materials, calculate the theoretical specific energy (Wh/kg) of the vanadium flow battery. (81/3 marks) The actual specific energy of a practical vanadium flow battery is typically approximately 20 Wh/kg. Explain why the actual specific energy of a practical vanadium flow battery is always significantly lower than the theoretical specific energy value based on the active masses of the materials. Give at least two reasons. (3 marks)
Step by Step Solution
★★★★★
3.36 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
1 At standard conditions E Ecathode E anode 099 026 125 n 2 F 96500 AG nF E 2 x 96500 x 125 24125...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


