Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemistry Question 2 You are an engineer in a manufacturing plant. Your company will procure an important equipment that will contain and heat 1 0
Chemistry
Question
You are an engineer in a manufacturing plant. Your company will procure an important equipment that will contain and heat moles of ammonia gas. The initial condition of the ammonia gas will be at atm and liters. The ammonia gas will then be heated until it expands to liters while maintaining the atm pressure.
For this, a meeting with the accounting department is to be facilitated to talk about the cost of the said equipment.
A teammate of yours, an engineer and a previous classmate of yours, made hisher computations regarding the cost. You've found out that hisher computations are "ideal" that:
heshe used specific heat capacities intended only for deg C and kpa conditions and heshe used ideal gas law.
From your extensive training in engineering school, you know that specific heat capacity varies with temperature and instead of using ideal gas law, it is more realistic to use real gas law" such as Van Der Waals equation see presented formula
According to a research made by the RnD Research and Development department of your company, for every positive increase in the change of entropy in the process of the equipment to be procured the cost accounts for Php Thus, JK increase in entropy in the process of the said equipment corresponds to Php; and K increase corresponds to Php
Save your workmate from hisher impending trouble by showing himher the difference between the cost heshe computed ideal and yours realistic in Php
Should your workmate present hisher computations, the accounting department will process the release of the financing of the equipment which could take some time profitability reviews, signatories of various bosses, etc. Once the money is released, it would be a lot trouble if what heshe requested is way below what it is supposed to be
For the Van Der Waal's equation:
P atm
n mol
V L
T K
For ammonia with respect to the Van Der Waal's equation:
a Lmol
b Lmol
Formula to convert Celsius to Kelvin is given by:
Kdeg C
Answer Format: decimals
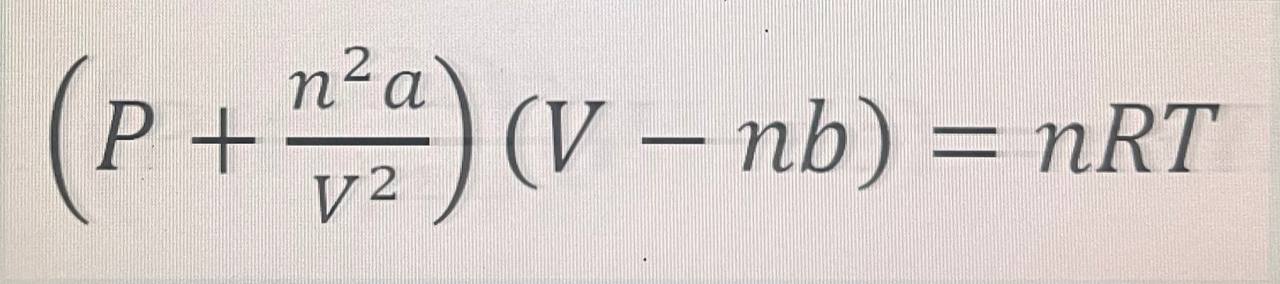
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


