Question
1) Consider barium sulfate crystals in saturated aqueous solution with interfacial energy of 120 mJ/m? and Gibbs free energy barrier of 5.02x1019 J a.
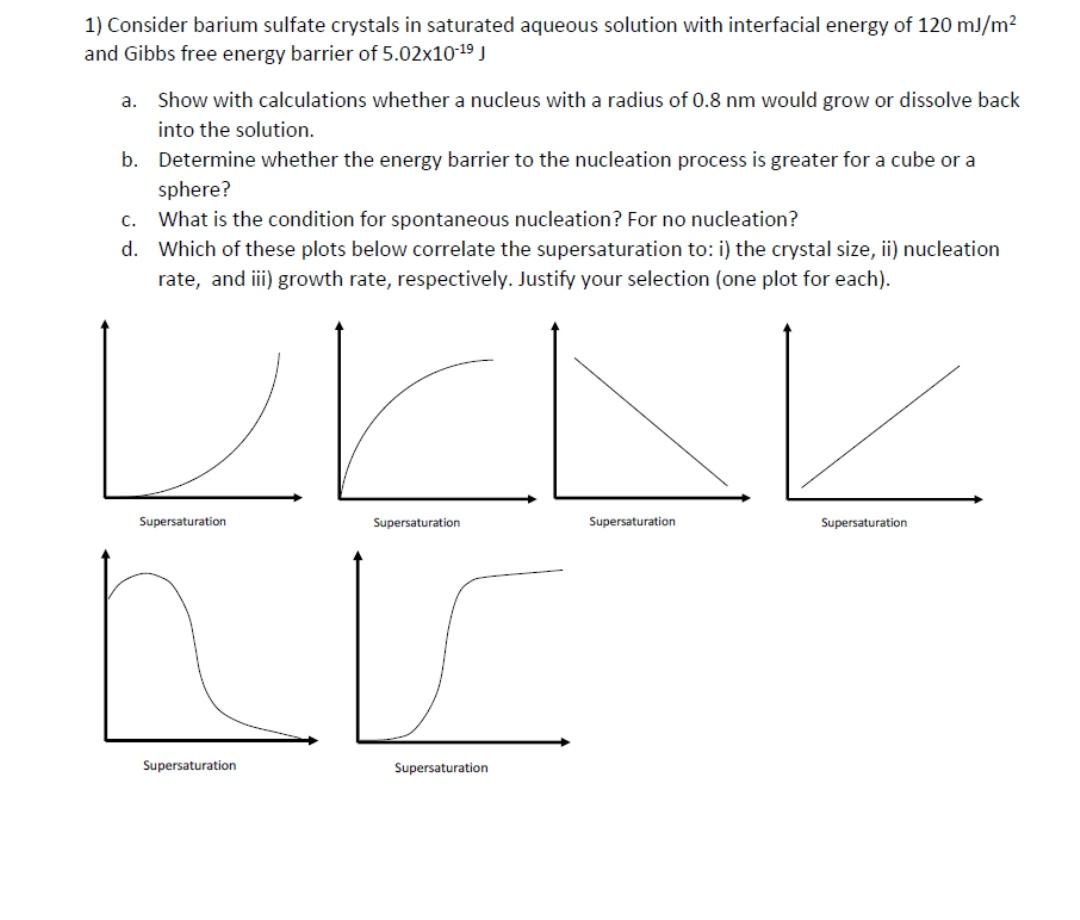
1) Consider barium sulfate crystals in saturated aqueous solution with interfacial energy of 120 mJ/m? and Gibbs free energy barrier of 5.02x1019 J a. Show with calculations whether a nucleus with a radius of 0.8 nm would grow or dissolve back into the solution. b. Determine whether the energy barrier to the nucleation process is greater for a cube or a sphere? c. What is the condition for spontaneous nucleation? For no nucleation? d. Which of these plots below correlate the supersaturation to: i) the crystal size, ii) nucleation rate, and i) growth rate, respectively. Justify your selection (one plot for each). Supersaturation Supersaturation Supersaturation Supersaturation Supersaturation Supersaturation
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



