Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Write the oxidation half-reaction, reduction half-reaction, and the net redox reaction between KMNO4 and FeCl,. Explain why the oxidation capability of permanganate increases in
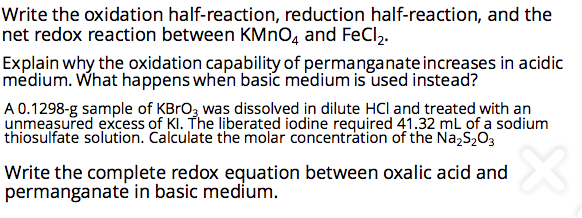
Write the oxidation half-reaction, reduction half-reaction, and the net redox reaction between KMNO4 and FeCl,. Explain why the oxidation capability of permanganate increases in acidic medium. What happens when basic medium is used instead? A 0.1298-g sample of KBrO3 was dissolved in dilute HCl and treated with an unmeasured excess of KI. The liberated iodine required 41.32 mL of a sodium thiosulfate solution. Calculate the molar concentration of the Na,S2O3 Write the complete redox equation between oxalic acid and permanganate in basic medium.
Step by Step Solution
★★★★★
3.57 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6362a968caf1d_236111.pdf
180 KBs PDF File
6362a968caf1d_236111.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


