Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chlorofluorcarbons (CFCs) are anthropogenic pollutants that persist in the stratosphere where they have been implicated in the depletion of the ozone layer. The chemical
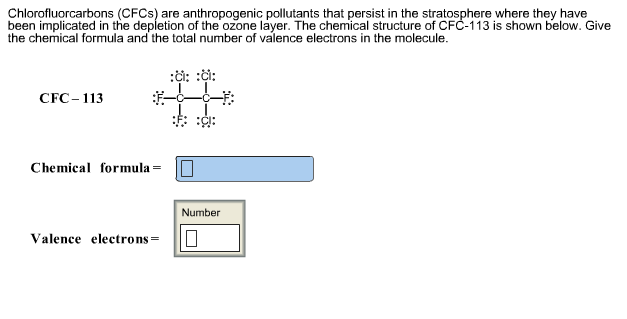
Chlorofluorcarbons (CFCs) are anthropogenic pollutants that persist in the stratosphere where they have been implicated in the depletion of the ozone layer. The chemical structure of CFC-113 is shown below. Give the chemical formula and the total number of valence electrons in the molecule. CFC-113 Chemical formula Valence electrons = :: :: Number 0 Dilorofluorcarbons (CFCs) are highly stable gas molecules that travel through the troposphere and deep into the stratosphere where they have contributed to the depletion of the ozone layer. To circumvent the harmful environmental effects of CFCs, chemists developed hydrochlorofluorocarbons (HCFCs). They are less harmful than CFCs because they decompose much more readily in the trophosphere than CFCs. Draw the Lewis dot structures for CFC-11 (CCI3F), CFC-12 (CClF2), and HCFC-22 (CHCIF2). CFC-11 CFC-12 HCFC22
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer The L...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


