Question
choose the best Lewis structure for seO4 49 & 50) Give the Lewis structure, bond angle, bonding pairs, lone pairs, electron geometry, molecular geometry for:
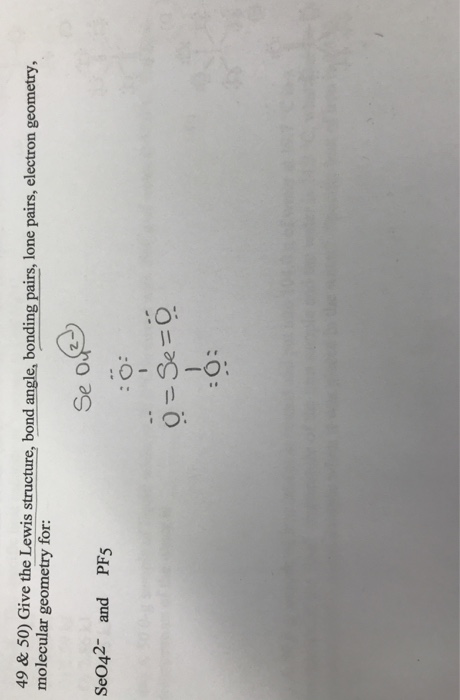
49 & 50) Give the Lewis structure, bond angle, bonding pairs, lone pairs, electron geometry, molecular geometry for: SeO42- and PF5 Se 10-8-6: 0= Se=0
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
Answer SeO o Number of Bond pairs bp 4 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



