Question
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Solutes Formula Hydrochloric acid HC1 Potassium hydroxide Formic
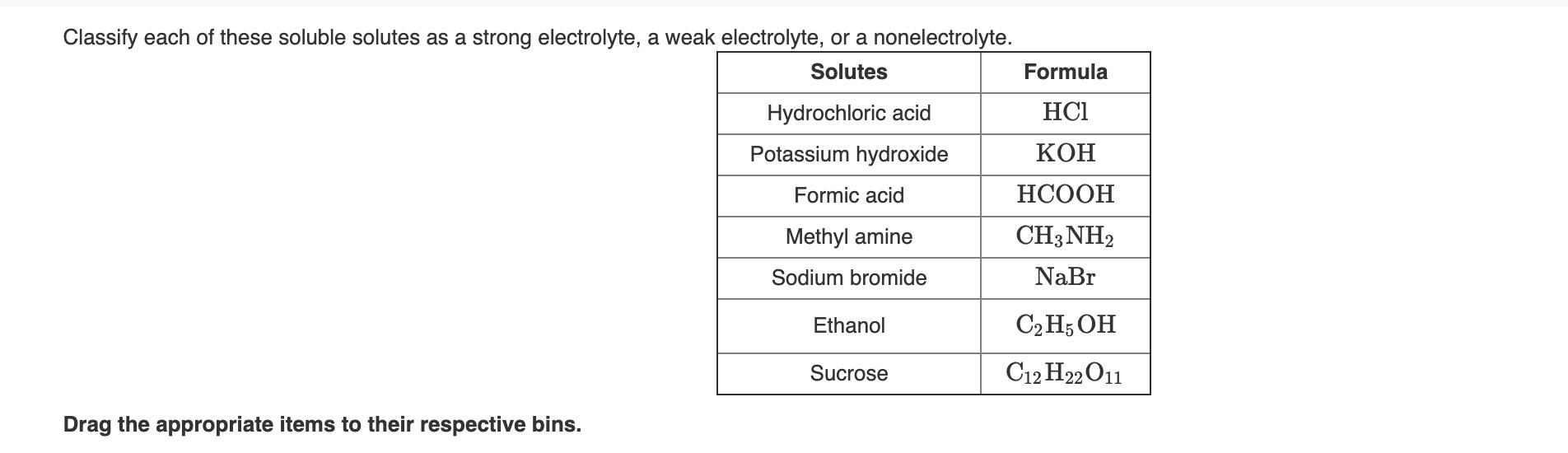
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Solutes Formula Hydrochloric acid HC1 Potassium hydroxide Formic acid Methyl amine CH3NH2 Sodium bromide NaBr Ethanol C2 H; OH Sucrose C12 H22 O11 Drag the appropriate items to their respective bins.
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Strong electrolyte 1Hydrochloric Acid HCL 2...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantum Chemistry
Authors: Ira N. Levine
7th edition
321803450, 978-0321803450
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



