Question
Classify each statement about effective nuclear charge, Zeff, as true or false. True Effective nuclear charge does not depend on the number of electrons
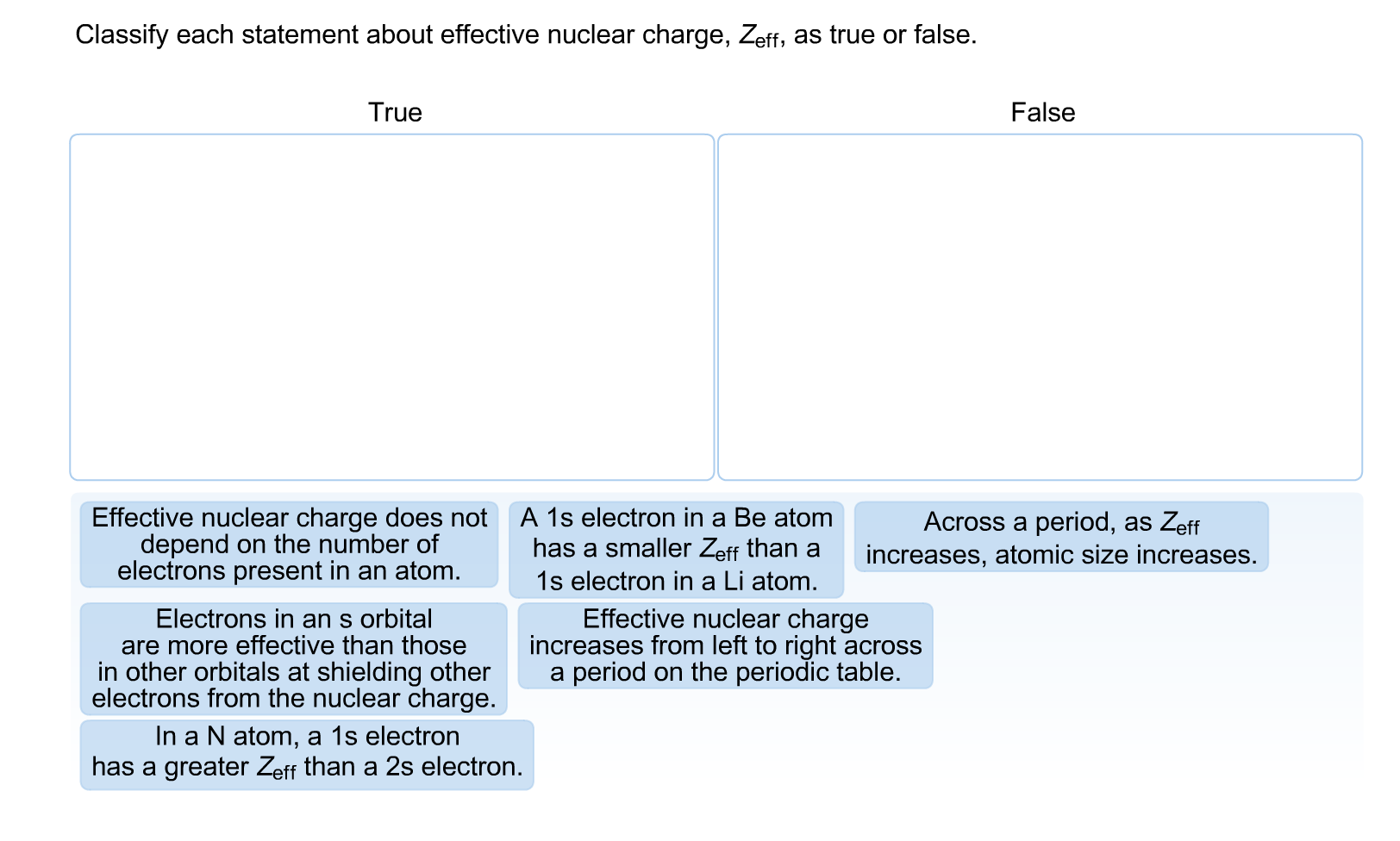
Classify each statement about effective nuclear charge, Zeff, as true or false. True Effective nuclear charge does not depend on the number of electrons present in an atom. Electrons in an s orbital are more effective than those in other orbitals at shielding other electrons from the nuclear charge. A 1s electron in a Be atom has a smaller Zeff than a 1s electron in a Li atom. In a N atom, a 1s electron has a greater Zeff than a 2s electron. False Across a period, as Zeff increases, atomic size increases. Effective nuclear charge increases from left to right across a period on the periodic table.
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Exploring Economics
Authors: Robert L Sexton
5th Edition
978-1439040249, 1439040249
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



