 classkick assessment i really need help on.
classkick assessment i really need help on.
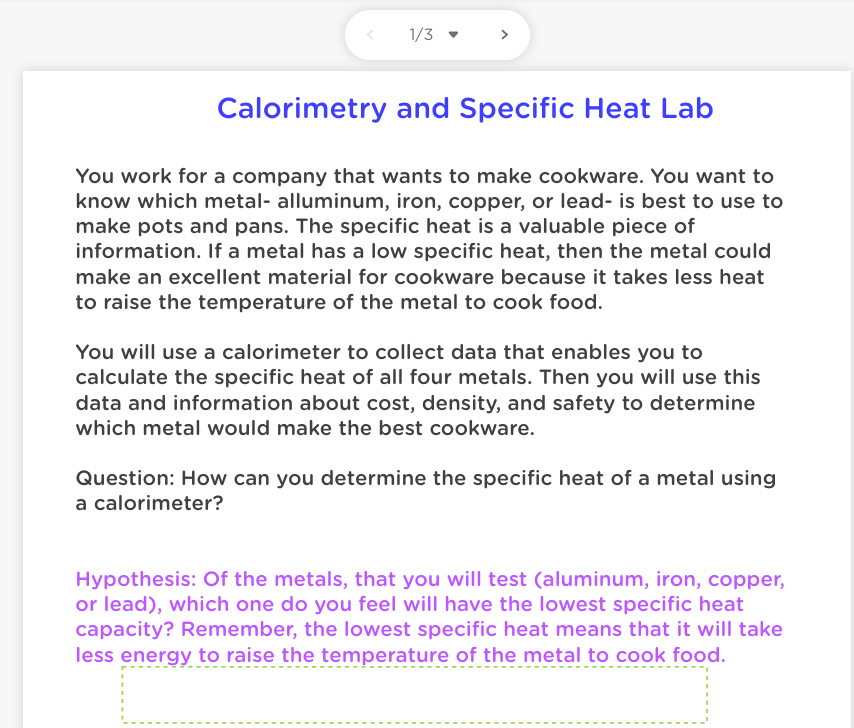
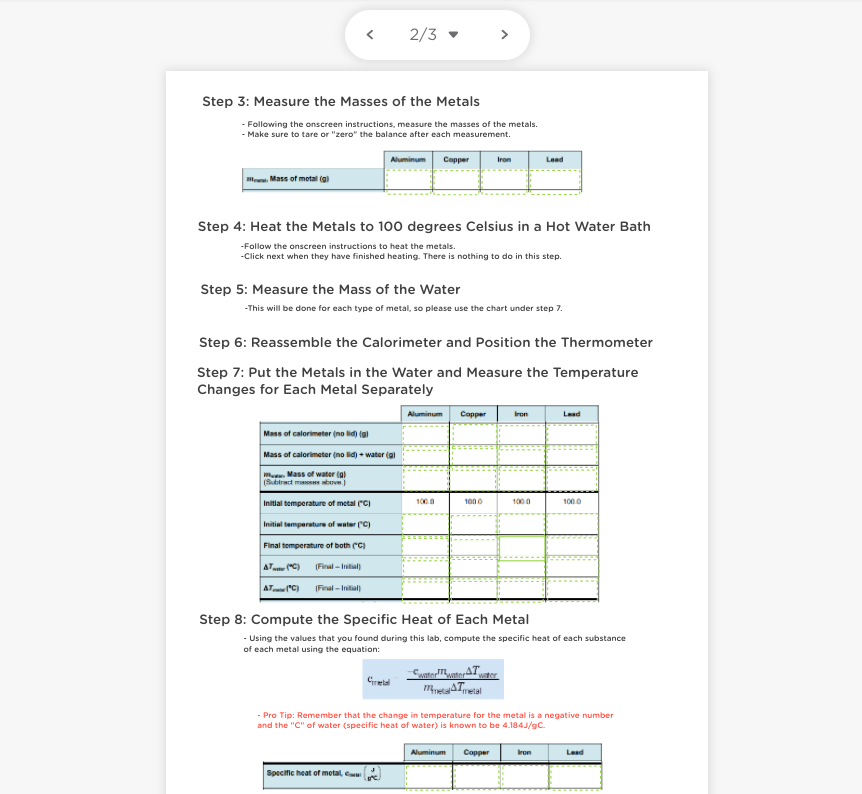
1/3 > Calorimetry and Specific Heat Lab You work for a company that wants to make cookware. You want to know which metal- alluminum, iron, copper, or lead- is best to use to make pots and pans. The specific heat is a valuable piece of information. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. You will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Then you will use this data and information about cost, density, and safety to determine which metal would make the best cookware. Question: How can you determine the specific heat of a metal using a calorimeter? Hypothesis: Of the metals, that you will test (aluminum, iron, copper, or lead), which one do you feel will have the lowest specific heat capacity? Remember, the lowest specific heat means that it will take less energy to raise the temperature of the metal to cook food. 2/3 > Step 3: Measure the masses of the Metals - Following the onscreen instructions, measure the masses of the metals. - Make sure to tare or "zero" the balance after each measurement. Aluminum Copper Iron Lead la Mass of metala Step 4: Heat the Metals to 100 degrees Celsius in a Hot Water Bath -Follow the onscreen instructions to heat the metals. -Click next when they have finished heating. There is nothing to do in this step. Step 5: Measure the mass of the Water -This will be done for each type of metal, so please use the chart under step 7. Step 6: Reassemble the Calorimeter and Position the Thermometer Step 7: Put the Metals in the Water and Measure the Temperature Changes for Each Metal Separately Aluminum Capper Lead Mass of calorimeter (no id) { Mass of calorimeter (no lid) + water (al Mass of waterial (Subiramo 1000 1000 1000 1000 Initial temperature of metal (C) Initial temperature of water (C) Final temperature of both (C A... (C) Final - Intl AT (C) Final - Initial Step 8: Compute the Specific Heat of Each Metal - Using the values that you found during this lab, compute the specific heat of each substance of each metal using the equation: Gretel Sto, AT Areal - Pro Tip: Remember that the change in temperature for the metal is a negative number and the "C" of water (specific heat of water) is known to be 4.184J/9C. Aluminum Copper bron Lead Specific heat of metal, 3/3 - Considering only specific heat, which metal would be the most ideal for use in cookware? Why? Looking at the factors of specific heat, cost, density (which can indicate how heavy it is), and safety risks, which metal would be the most ideal for use in cookware? Why? Aluminum Copper Iron Lead Cost of metal Safety risk $1.00 pound $5.00 pound 30.10pound $1.00 pound Slight: Slight: None Significant corrodes tomic at high Source of toxic when casily when concentration: beneficial ingested exposed to can bach dietary iron can leach from acidic foods pans 2.70 8.92 7.87 113 Density of metal (gim You are done with the Lab! Hooray!!! Make sure to submit a blank document titled "Classkick" into the dropbox. W 1/3 > Calorimetry and Specific Heat Lab You work for a company that wants to make cookware. You want to know which metal- alluminum, iron, copper, or lead- is best to use to make pots and pans. The specific heat is a valuable piece of information. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. You will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Then you will use this data and information about cost, density, and safety to determine which metal would make the best cookware. Question: How can you determine the specific heat of a metal using a calorimeter? Hypothesis: Of the metals, that you will test (aluminum, iron, copper, or lead), which one do you feel will have the lowest specific heat capacity? Remember, the lowest specific heat means that it will take less energy to raise the temperature of the metal to cook food. 2/3 > Step 3: Measure the masses of the Metals - Following the onscreen instructions, measure the masses of the metals. - Make sure to tare or "zero" the balance after each measurement. Aluminum Copper Iron Lead la Mass of metala Step 4: Heat the Metals to 100 degrees Celsius in a Hot Water Bath -Follow the onscreen instructions to heat the metals. -Click next when they have finished heating. There is nothing to do in this step. Step 5: Measure the mass of the Water -This will be done for each type of metal, so please use the chart under step 7. Step 6: Reassemble the Calorimeter and Position the Thermometer Step 7: Put the Metals in the Water and Measure the Temperature Changes for Each Metal Separately Aluminum Capper Lead Mass of calorimeter (no id) { Mass of calorimeter (no lid) + water (al Mass of waterial (Subiramo 1000 1000 1000 1000 Initial temperature of metal (C) Initial temperature of water (C) Final temperature of both (C A... (C) Final - Intl AT (C) Final - Initial Step 8: Compute the Specific Heat of Each Metal - Using the values that you found during this lab, compute the specific heat of each substance of each metal using the equation: Gretel Sto, AT Areal - Pro Tip: Remember that the change in temperature for the metal is a negative number and the "C" of water (specific heat of water) is known to be 4.184J/9C. Aluminum Copper bron Lead Specific heat of metal, 3/3 - Considering only specific heat, which metal would be the most ideal for use in cookware? Why? Looking at the factors of specific heat, cost, density (which can indicate how heavy it is), and safety risks, which metal would be the most ideal for use in cookware? Why? Aluminum Copper Iron Lead Cost of metal Safety risk $1.00 pound $5.00 pound 30.10pound $1.00 pound Slight: Slight: None Significant corrodes tomic at high Source of toxic when casily when concentration: beneficial ingested exposed to can bach dietary iron can leach from acidic foods pans 2.70 8.92 7.87 113 Density of metal (gim You are done with the Lab! Hooray!!! Make sure to submit a blank document titled "Classkick" into the dropbox. W


 classkick assessment i really need help on.
classkick assessment i really need help on.





