Answered step by step
Verified Expert Solution
Question
1 Approved Answer
clearer pictures :) 5. (12pts) Interpret the mass spectrum for 1-hexanol by identifying the major fragments listed below. Attach the mass spectrum for 1-hexanol to
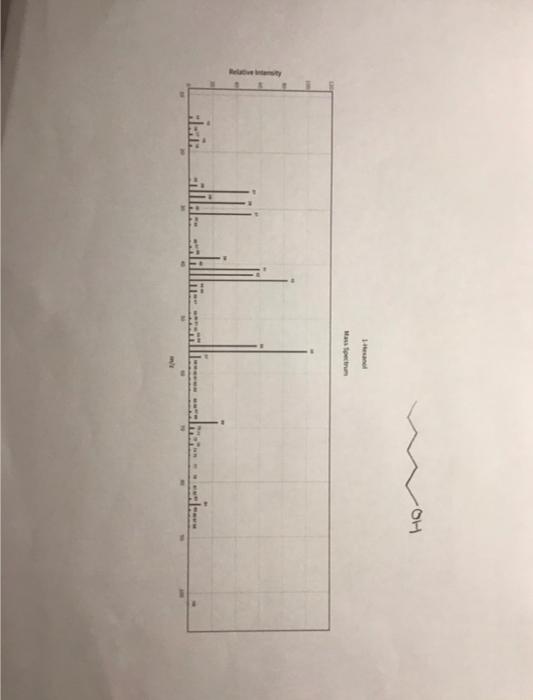

clearer pictures :) 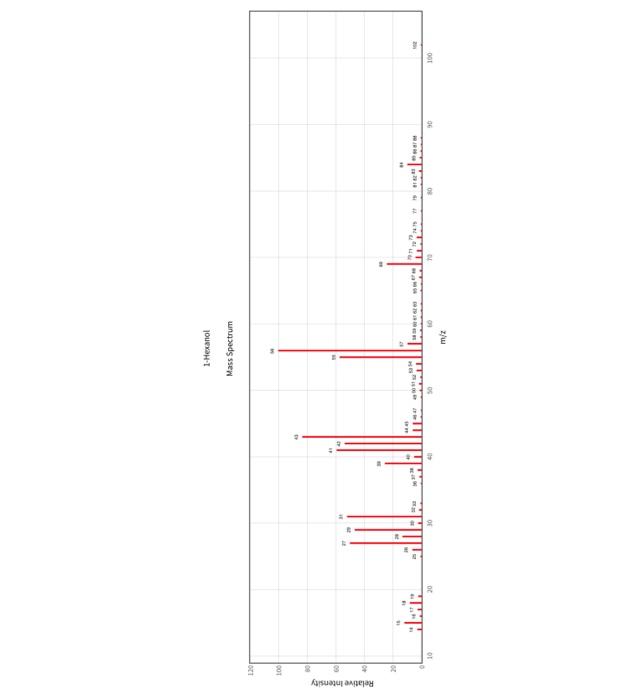

5. (12pts) Interpret the mass spectrum for 1-hexanol by identifying the major fragments listed below. Attach the mass spectrum for 1-hexanol to this assignment. a. Draw the structure of the molecular ion for 1-hexanol. Is it present in the spectrum? If so identify the peak which correlates to the molecular ion. b. Draw the structure of the fragment (cation or radical cation) that forms when 1-hexanol undergoes cleavage. Show the resonance stabilization of the fragment. Is it present in the spectrum? If so identify the peak which correlates to the alpha cleavage fragment. c. Draw the structure of the fragment (cation or radical cation) that forms when 1-hexanol undergoes the loss of water. Is it present in the spectrum? If so identify the peak which correlates to the dehydrated fragment. d. The dehydrated fragment can be cleaved to form an allylic cation. Draw the structure of the allylic cation fragment. Is it present in the spectrum? If so identify the peak which correlates to the allylic cation fragment. 6. (12pts) Interpret the mass spectrum for 2-hexanol by identifying the major fragments listed below. Attach the mass spectrum for 2-hexanol to this assignment. a. Draw the structure of the molecular ion for 2-hexanol. Is it present in the spectrum? If so identify the peak which correlates to the molecular ion. 5. (12pts) Interpret the mass spectrum for 1-hexanol by identifying the major fragments listed below. Attach the mass spectrum for 1-hexanol to this assignment. a. Draw the structure of the molecular ion for 1-hexanol. Is it present in the spectrum? If so identify the peak which correlates to the molecular ion. b. Draw the structure of the fragment (cation or radical cation) that forms when 1-hexanol undergoes cleavage. Show the resonance stabilization of the fragment. Is it present in the spectrum? If so identify the peak which correlates to the alpha cleavage fragment. c. Draw the structure of the fragment (cation or radical cation) that forms when 1-hexanol undergoes the loss of water. Is it present in the spectrum? If so identify the peak which correlates to the dehydrated fragment. d. The dehydrated fragment can be cleaved to form an allylic cation. Draw the structure of the allylic cation fragment. Is it present in the spectrum? If so identify the peak which correlates to the allylic cation fragment. 6. (12pts) Interpret the mass spectrum for 2-hexanol by identifying the major fragments listed below. Attach the mass spectrum for 2-hexanol to this assignment. a. Draw the structure of the molecular ion for 2-hexanol. Is it present in the spectrum? If so identify the peak which correlates to the molecular ion 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


