Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Activity 2: Effect of solute concentration on cell membranes For this activity, you will investigate what happens to red blood cells exposed to different
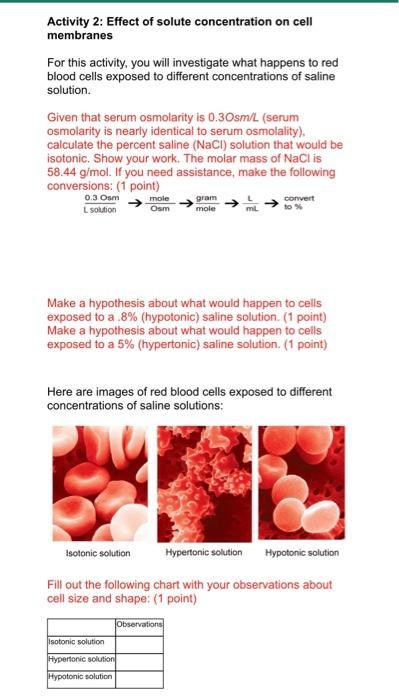
Activity 2: Effect of solute concentration on cell membranes For this activity, you will investigate what happens to red blood cells exposed to different concentrations of saline solution. Given that serum osmolarity is 0.30sm/L (serum osmolarity is nearly identical to serum osmolality), calculate the percent saline (NaCl) solution that would be isotonic. Show your work. The molar mass of NaCl is 58.44 g/mol. If you need assistance, make the following conversions: (1 point) 0.3 Osm L solution mole Osm gram mole Isotonic solution L mL Make a hypothesis about what would happen to cells exposed to a .8% (hypotonic) saline solution. (1 point) Make a hypothesis about what would happen to cells exposed to a 5% (hypertonic) saline solution. (1 point) Isotonic solution Hypertonic solution Hypotonic solution convert to % Here are images of red blood cells exposed to different concentrations of saline solutions: Hypertonic solution Hypotonic solution Fill out the following chart with your observations about cell size and shape: (1 point) Observations
Step by Step Solution
★★★★★
3.47 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Calculating Percent Saline Solution To calculate the percent saline solution that would be isotonic we will use the following formula Molarity Moles of soluteVolume of Solution x 1000 Given that the s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


