Question
1. The figure below shows air at steady state, entering a nozzle at a pressure of 250 kPa and a temperature of 77C with
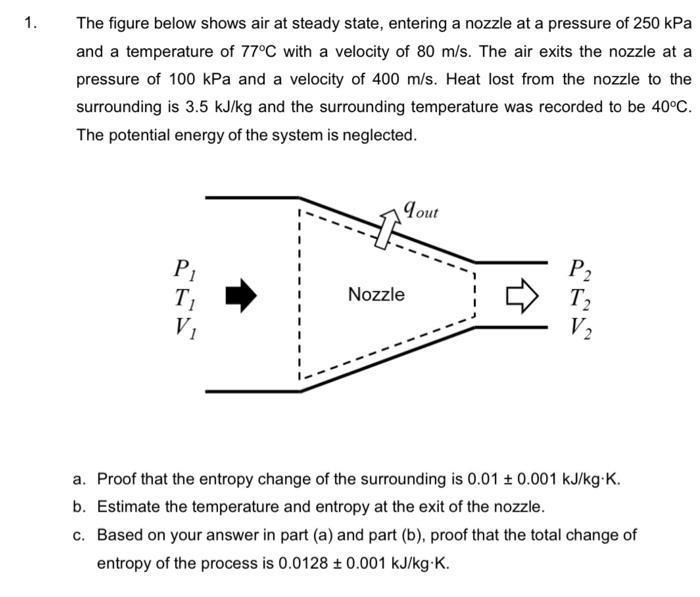
1. The figure below shows air at steady state, entering a nozzle at a pressure of 250 kPa and a temperature of 77C with a velocity of 80 m/s. The air exits the nozzle at a pressure of 100 kPa and a velocity of 400 m/s. Heat lost from the nozzle to the surrounding is 3.5 kJ/kg and the surrounding temperature was recorded to be 40C. The potential energy of the system is neglected. P T V qout Nozzle P2 T V a. Proof that the entropy change of the surrounding is 0.01 0.001 kJ/kg-K. b. Estimate the temperature and entropy at the exit of the nozzle. c. Based on your answer in part (a) and part (b), proof that the total change of entropy of the process is 0.0128 0.001 kJ/kg-K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution a P 250 kpa T 77C 350k V 80 m8 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



