Question
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific
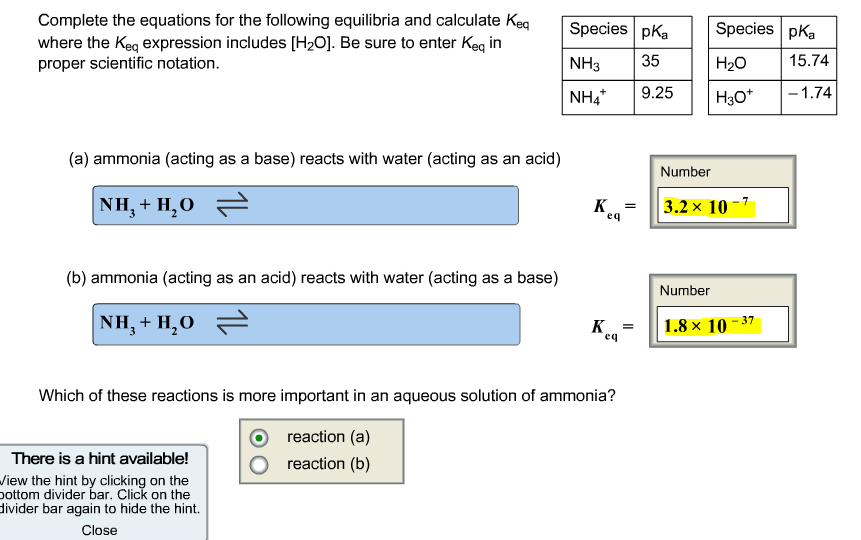
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts with water (acting as an acid) NH,+H,O = (b) ammonia (acting as an acid) reacts with water (acting as a base) NH,+H,O = Species pKa 35 9.25 There is a hint available! View the hint by clicking on the bottom divider bar. Click on the divider bar again to hide the hint. Close NH3 NH4* K eq K eq Which of these reactions is more important in an aqueous solution of ammonia? reaction (a) reaction (b) = Species pKa HO H3O+ Number 3.2x 10-7 Number 1.8 10-37 15.74 -1.74
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Accounting A Practical Approach
Authors: Jeffrey Slater
12th edition
978-0132772068, 133468100, 013277206X, 9780133468106, 978-0133133233
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



