Answered step by step
Verified Expert Solution
Question
1 Approved Answer
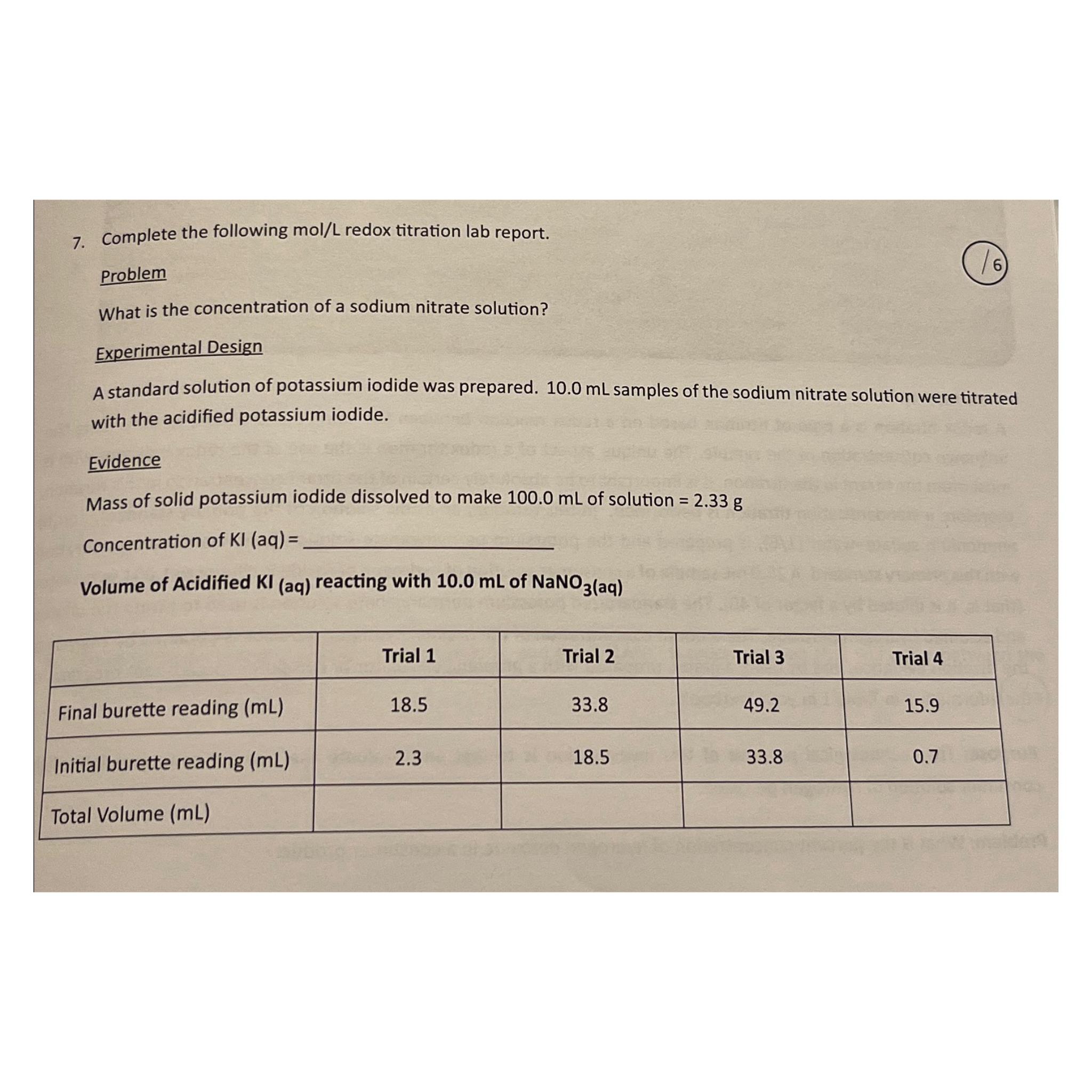
Complete the following m o l L redox titration lab report. Problem What is the concentration of a sodium nitrate solution? Experimental Design A standard
Complete the following redox titration lab report.
Problem
What is the concentration of a sodium nitrate solution?
Experimental Design
A standard solution of potassium iodide was prepared. samples of the sodium nitrate solution were titrated with the acidified potassium iodide.
Evidence
Mass of solid potassium iodide dissolved to make of solution
Concentration of
Volume of Acidified aq reacting with of aq
tableTrial Trial Trial Trial Final burette reading Initial burette reading Total Volume
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


