Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Complete the following structural formula for a neutral molecule by adding H atoms to complete the valence of each atom. Do not introduce any double
Complete the following structural formula for a neutral molecule
by adding atoms to complete the valence of each atom. Do not
introduce any double or triple bonds.
Then write the molecular formula in the order CHO.
Complete the following structural formula for a neutral molecule
by adding atoms to complete the valence of each atom. Do not
introduce any double or triple bonds.
Then complete the Lewis diagram by adding any unshared electron
pairs needed, so that each atom except has a complete octet.
Write the molecular formula in the order CHO.
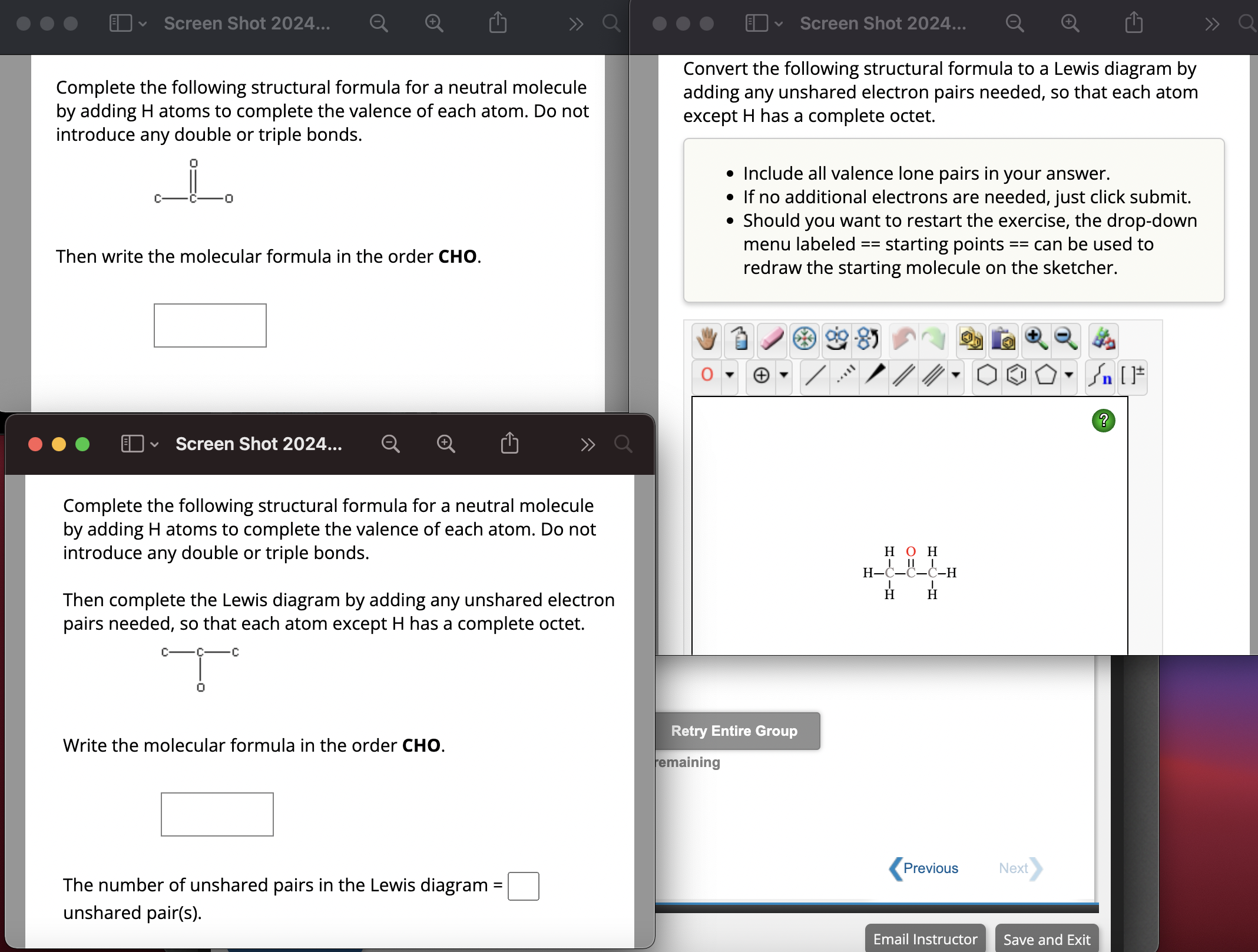
Convert the following structural formula to a Lewis diagram by
adding any unshared electron pairs needed, so that each atom
except has a complete octet.
Include all valence lone pairs in your answer.
If no additional electrons are needed, just click submit.
Should you want to restart the exercise, the dropdown
menu labeled starting points can be used to
redraw the starting molecule on the sketcher.
The number of unshared pairs in the Lewis diagram
unshared pairs
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


